Synthesis of novel imidazole-based triheterocycles via a domino Ugi/Michael reaction and silver-catalyzed heteroannulation†
Zhenghua Li‡
a,
Yaping Zhao‡ab,
Guilong Tiana,
Yi Hea,
Gonghua Songab,
Luc Van Meervelt c and
Erik V. Van der Eycken*a
c and
Erik V. Van der Eycken*a
aLaboratory for Organic & Microwave-Assisted Chemistry (LOMAC), University of Leuven (KU Leuven), Celestijnenlaan 200F, B-3001 Leuven, Belgium. E-mail: erik.vandereycken@chem.kuleuven.be
bShanghai Key Laboratory of Chemical Biology, East China University of Science and Technology, Shanghai 200237, People's Republic of China
cBiomolecular Architecture, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, B-3001, Leuven, Belgium
First published on 26th October 2016
Abstract
An efficient and diversity-oriented synthetic strategy for novel triheterocyclic imidazo-pyrrolo-pyrazine/diazepine/diazocine derivatives is elaborated. The process utilizes a domino four-component Ugi reaction/Michael reaction of imidazole-2-carbaldehyde, propargyl amines, 2-alkynoic acids and isonitriles to deliver functionalized imidazole-pyrrolones, which upon a silver-catalyzed heteroannulation in aqueous medium under microwave irradiation furnishes the desired triheterocycles in good to excellent yields.
Introduction
Nitrogen-containing polyheterocycles are widely present in biologically active natural products1 and pharmaceuticals.2 Among them, substituted imidazoheterocyclic skeletons fused with pyrrolone or pyrazines/diazepines/diazocines represent a class of interesting target molecules due to their potential biological activities such as modulation of the cannabinoid receptors,3 inhibition of the integrin receptor,4 antagonist of vasopressin and oxytocin,5 anticancer,6 antiviral7 and insecticidal activities.8 However, most of the synthetic routes for polyheterocycles are generally restricted by a multistep procedure, and the preparation of highly functionalized precursors,9 driving the synthetic community to develop short and efficient synthetic sequences (Fig. 1).Isocyanide-based multicomponent reactions (MCRs) have gained considerable popularity for the synthesis of substituted imidazole-based heterocycles.10 Moreover, the advent of transition-metal catalyzed post-multicomponent reactions (MCR) offers new pathways for the diversity-oriented construction of complex bioactive heterocycles in a few steps.11 Due to their remarkable ability to activate π-systems, especially alkynes, gold12 and silver13 catalysts are widely used for intramolecular heteroannulation and hydroarylation processes to construct carbo- and heterocyclic compounds. In this context, our group has explored several sequential Ugi-four components reaction/transition metal-catalyzed intramolecular heteroannulation and hydroarylation approaches to assemble highly diversified heterocycles such as indolazocines,14 spiroindolines,15 imidazodiazepinones16 pyrroloazepinones and pyrrolopyridinones.17 Furthermore, the employment of water as solvent for microwave-assisted metal-catalyzed transformations,18 possesses many advantages like shortened reaction times, increased yields and cleaner processes.
Motivated by the interesting bioactivity of various imidazole-based triheterocycles and as a result of our continuous interest in designing new post-MCR strategies for the generation of novel heterocycles from readily available starting materials, we anticipated that a facile synthesis of functionalized triheterocyclic imidazo-pyrrolo-pyrazines/diazepines/diazocines via a domino Ugi/Michael reaction and subsequent silver-catalyzed heteroannulation would be quite promising (Scheme 1).
Results and discussion
To test the feasibility of our hypotheses, a model substrate model substrate was synthesized via Ugi-4CR19 of imidazole-2-carbaldehyde (1a), propargyl amine (2a), 2-butynoic acid (3a) and tert-butyl isonitrile (4a) (Table 1). Much to our delight, instead of the formation of the Ugi adduct, this process furnished immediately the imidazo-pyrrolone 5a in 72% yield through a domino Ugi/Michael reaction.20 Then, the intramolecular heteroannulation of the adduct 5a was explored. This reaction proceeded smoothly in the presence of Au(PPh3)OTf (5 mol%) in chloroform at 70 °C for 4 h affording the desired triheterocycle 6a in an excellent yield of 98% with completely exo-dig selectivity (Table 1, entry 1). Similarly, when different silver salts like AgOTf, AgBF4, AgNTf2, AgSbF6 were used, equally excellent yields were obtained (Table 1, entries 2–5). While with AuCl3, AuCl and CuCl a slight decreased yield was observed (Table 1, entries 6–8), the reaction met with failure when InCl3 or PtCl2 were used (Table 1, entries 9 and 10). The comparatively cheaper and similarly efficient AgSbF6 was selected to examine the solvent effect. The intramolecular heteroannulation went well in solvents like toluene, THF, MeOH and water (Table 1, entries 11–14). Considering the environmental benign issue and the advantages of microwave-assisted metal-catalyzed transformations in aqueous medium, this transformation was performed under microwave irradiation in water at 120 °C for 0.5 h resulting in the formation of 6a in 89% yield (Table 1, entry 15). When performed under nitrogen atmosphere, 6a was obtained in an excellent yield of 98% (Table 1, entry 16). A lower yield was observed when the catalyst loading was diminished to 2 mol% (Table 1, entry 17).| Entry | Catalyst | Solvent | T/°C | Time/h | Yieldb of (±)-6a/% |
|---|---|---|---|---|---|
| a All the reactions were run on 0.05 mmol scale of 5a with catalyst (5 mol%), solvent (1 mL).b Isolated yields.c The reaction was run under microwave irradiation at a maximum power of 100 W.d Under N2.e 2 mol% of AgSbF6 was used. | |||||
| 1 | Au(PPh3)OTf | CHCl3 | 70 | 4 | 98 |
| 2 | AgOTf | CHCl3 | 70 | 4 | 94 |
| 3 | AgBF4 | CHCl3 | 70 | 4 | 96 |
| 4 | AgNTf2 | CHCl3 | 70 | 4 | 92 |
| 5 | AgSbF6 | CHCl3 | 70 | 4 | 98 |
| 6 | AuCl3 | CHCl3 | 70 | 4 | 88 |
| 7 | AuCl | CHCl3 | 70 | 4 | 84 |
| 8 | CuCl | CHCl3 | 70 | 4 | 81 |
| 9 | InCl3 | CHCl3 | 70 | 4 | 18 |
| 10 | PtCl2 | CHCl3 | 70 | 4 | 0 |
| 11 | AgSbF6 | Toluene | 70 | 4 | 98 |
| 12 | AgSbF6 | THF | 70 | 4 | 98 |
| 13 | AgSbF6 | MeOH | 70 | 4 | 94 |
| 14 | AgSbF6 | H2O | 110 | 8 | 96 |
| 15 | AgSbF6 | H2O | 120c | 0.5 | 89 |
| 16d | AgSbF6 | H2O | 120c | 0.5 | 98 |
| 17d,e | AgSbF6 | H2O | 120c | 0.5 | 62 |
With the optimized reaction conditions in hand (Table 1, entry 14), we synthesized diversely substituted Ugi/Michael adducts 5b–o to evaluate the scope and limitations for this regioselective intramolecular heteroannulation process (Table 2). Initial exploration of the scope focused on varying the substitution pattern of 2-alkynoic acid and the isonitrile. Pleasingly, the heteroannulation proceeded smoothly to deliver the imidazo-pyrrolo-pyrazines 6b–j in good to excellent yields. Due to the comparatively poor solubility of the adduct 5j bearing a phenyl substituent on the initial 2-alkynoic acid, a lower yield was observed in the formation of 6j. We next examined the influence of the substituent on propargyl amine. A disubstitution pattern on C-1 was well tolerated yielding the corresponding triheterocycles 6k and 6l in 85% and 55% respectively. The optimized conditions failed to give the desired triheterocycles in the case of Ugi/Michael adducts 5m–o derived from substituted propargyl amines bearing a phenyl substituent on the terminal alkyne, while performing these reactions in chloroform at 70 °C for 12 h give 6m–o in good yields. Additionally, the tricyclic skeleton and geometrical orientations of compound 6a was unambiguously confirmed by X-ray crystallography (Fig. 2).
| a The reaction were run on a 0.1 mmol scale of 5a–o with AgSbF6 (5 mol%), and H2O (2 mL) in a screw capped vial at 120 °C for 0.5 h under microwave irradiation at a maximum power of 100 W.b The reactions were performed on a 0.1 mmol scale of 5m–o with AgSbF6 (5 mol%) and CHCl3 (2 mL) in a screw capped vial at 70 °C for 12 h in an oil bath. TMB = 1,1,3,3-tetramethylbutyl, Cp = cyclopentane. |
|---|
 |
 | ||
| Fig. 2 Crystal structure of compound 6a with thermal ellipsoids set at the 50% probability level.21 | ||
To our satisfaction, the scope of the protocol could be extended to imidazo-pyrrolo-diazepines and -diazocines. The Ugi/Michael adducts 5p–u were prepared from 3-butyn-1-amine or 4-pentyn-1-amine, and subjected to the optimized conditions. The Ag(I)-catalyzed exo-dig cyclization proceeded smoothly resulting in the formation of the triheterocyclic imidazo-pyrrolo-diazepines 6p–r and imidazo-pyrrolo-diazocines 6s–u in moderate to good yield (Table 3). It is notable that the imidazo-pyrrolone-diazocines 6s–u are generated in a lower yields because of enthalpic and entropic reasons in the formation of eight-membered ring.22
| a All reactions were run on a 0.1 mmol scale of 5p–u with AgSbF6 (5 mol%) and H2O (2 mL) in a screw capped vial at 120 °C for 0.5 h under microwave irradiation at a maximum power of 100 W. TMB = 1,1,3,3-tetramethylbutyl. |
|---|
 |
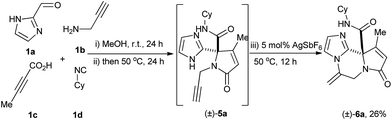
We also evaluated the possibility to run the sequence as a one-pot-three-step fashion. After the Ugi-4CR was performed at r.t. for 24 h, the reaction mixture was heated at 50 °C for 24 h. Subsequently 5 mol% of AgSbF6 was added and the reaction mixture was heated at 50 °C for 12 h. The desired triheterocycles 6a was generated in an overall yield of 26% (Scheme 2).
Based on previous investigations,13,16,23 a plausible mechanism could be proposed (Scheme 3). The generated Ugi adduct 5a′ is undergoing a Michael addition to give the adduct 5a.20 Ag(I)-mediated heteroannulation of 5a delivers intermediate B via an exo-dig process. Finally, deprotonation and protodeauration leads to the formation of the triheterocycle 6a with concomitant regeneration of the silver catalyst.
Conclusions
In summary, we have elaborated an efficient and green methodology for the rapid construction of novel triheterocyclic imidazo-pyrrolo-pyrazines/diazepines/diazocines starting from readily available building blocks. The domino Ugi-4CR/Michael addition as the first step generates the diversity while the subsequent microwave-assisted Ag(I)-catalyzed heteroannulation in aqueous medium represents an efficient and green protocol resulting in the formation of the new triheterocyclic scaffolds.Acknowledgements
The authors wish to thank the FWO [Fund for Scientific Research-Flanders (Belgium)] and the Research Fund of the University of Leuven (KU Leuven) for financial support. LVM thank the Hercules Foundation for supporting the purchase of the diffractometer through project AKUL/09/0035. ZL, YZ, GT and YH appreciate the China Scholarship Council (CSC) for providing the financial support in their doctoral studies.Notes references
- (a) B. Forte, B. Malgesini, C. Piutti, F. Quartieri, A. Scolaro and G. Papeo, Mar. Drugs, 2009, 7, 705 CrossRef CAS PubMed; (b) M. Menna, E. Fattorusso and C. Imperatore, Molecules, 2011, 16, 8694 CrossRef CAS; (c) D. Fernández, A. Ahaidar, G. Danelón, P. Cironi, M. Marfil, O. Pérez, C. Cuevas, F. Albericio, J. A. Joule and M. Álvarez, Monatsh. Chem., 2004, 135, 615 CrossRef.
- (a) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed; (b) M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265 CrossRef PubMed.
- S. J. O'Connor, J. S. NeWcom, J. L. Ralbovsky, G. R. Gustafson, R. P. Beckett and R. Zhiyong, US Pat., 20120015930, 2012.
- B. K. Blackburn, K. Robarge and T. C. Somers, US Pat., 5705890, 1998.
- (a) J. D. Albngiht, M. F. Reich, F. Sum and E. G. D. Santos, US Pat., 5624923, 1997; (b) J. D. Albright, F. Sum and X. Du, US Pat., 5512563, 1996.
- T. B. Gordon and B. A. Morgan, WO Pat., 00/39130, 2000.
- R. C. Griffith, C. D. Roberts, F. U. Schmitz, J. Botyanszki, R. E. Marek, D. Shi and S. M. Pham, US Pat., 20080045498, 2008.
- J. T. Kim, J. Butt and V. Gevorgyan, J. Org. Chem., 2004, 69, 5638 CrossRef CAS PubMed.
- (a) K. C. Nicolaou, C. R. H. Hale, C. Nilewski and H. A. Ioannidou, Chem. Soc. Rev., 2012, 41, 5185 RSC; (b) I. S. Young, P. D. Thornton and A. Thompson, Nat. Prod. Rep., 2010, 27, 1801 RSC; (c) C. Cordier, D. Morton, S. Murrison, A. Nelson and C. O'Leary-Steele, Nat. Prod. Rep., 2008, 25, 719 RSC; (d) J. E. Sears and D. L. Boger, Acc. Chem. Res., 2015, 48, 653 CrossRef CAS PubMed; (e) P. Korakas, St. Chaffee, J. B. Shotwell, P. Duque and J. L. Wood, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12054 CrossRef CAS PubMed; (f) H. Kim, T. T. Tung and S. B. Park, Org. Lett., 2013, 15, 5814 CrossRef CAS PubMed; (g) L. Meng, J. C. Fettinger and M. J. Kurth, Org. Lett., 2007, 9, 5055 CrossRef CAS PubMed.
- (a) H. Zhou, W. Wang, O. Khorev, Y. Zhang, Z. Miao, T. Meng and J. Shen, Eur. J. Org. Chem., 2012, 28, 5585 CrossRef; (b) Z. Tber, M.-A. Hiebel, A. E. Hakmaoui, M. Akssira, G. Guillaumet and S. Berteina-Raboin, J. Org. Chem., 2015, 80, 6564 CrossRef CAS PubMed; (c) A. Sagar, V. N. Babu and D. S. Sharada, RSC Adv., 2015, 5, 29066 RSC; (d) S. Sadjadi, M. M. Heravi and N. Nazari, RSC. Adv., 2016, 6, 5320 RSC.
- (a) D. M. D'Souza and T. J. J. Müller, Chem. Soc. Rev., 2007, 36, 1095 RSC; (b) J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300 CrossRef CAS PubMed; (c) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed; (d) U. K. Sharma, N. Sharma, D. D. Vachhani and E. V. Van der Eycken, Chem. Soc. Rev., 2015, 44, 1836 RSC; (e) Z. Li, L. Legras, A. Kumar, D. D. Vachhani, S. K. Sharma, V. S. Parmar and E. V. Van der Eycken, Tetrahedron Lett., 2014, 55, 2070 CrossRef CAS; (f) N. Sharma, Z. Li, U. K. Sharma and E. V. Van der Eycken, Org. Lett., 2014, 16, 3884 CrossRef CAS PubMed; (g) Z. Li, N. Sharma, U. K. Sharma, J. Jacobs, L. Van Meervelt and E. V. Van der Eycken, Chem. Commun., 2016, 52, 5516 RSC.
- (a) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (b) H. C. Shen, Tetrahedron, 2008, 64, 3885 CrossRef CAS; (c) H. C. Shen, Tetrahedron, 2008, 64, 7847 CrossRef CAS; (d) A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766 RSC; (e) M. Rudolph and A. S. K. Hashmi, Chem. Soc. Rev., 2012, 41, 2448 RSC; (f) C. Ferrer and A. M. Echavarren, Angew. Chem., Int. Ed., 2006, 45, 1105 CrossRef CAS PubMed; (g) C. Ferrer, C. H. M. Amijs and A. M. Echavarren, Chem.–Eur. J., 2007, 13, 1358 CrossRef CAS PubMed.
- (a) J. Weibel, A. Blanc and P. Pale, Chem. Rev., 2008, 108, 3149 CrossRef CAS PubMed; (b) Y. Yamamoto, Chem. Rev., 2008, 108, 3199 CrossRef CAS PubMed; (c) M. Álvarez-Corral, M. Muñoz-Dorado and I. Rodríguez-García, Chem. Rev., 2008, 108, 3174 CrossRef PubMed.
- (a) S. G. Modha, D. D. Vachhani, J. Jacobs, L. Van Meervelt and E. V. Van der Eycken, Chem. Commun., 2012, 48, 6550 RSC; (b) A. Kumar, Z. Li, S. K. Sharma, V. S. Parmar and E. V. Van der Eycken, Chem. Commun., 2013, 49, 6803 RSC; (c) Z. Li, A. Kumar, D. D. Vachhani, S. K. Sharma, V. S. Parmar and E. V. Van der Eycken, Eur. J. Org. Chem., 2014, 2084 CrossRef CAS.
- (a) S. G. Modha, A. Kumar, D. D. Vachhani, J. Jacobs, S. K. Sharma, V. S. Parmar, L. Van Meervelt and E. V. Van der Eycken, Angew. Chem., Int. Ed., 2012, 51, 9572 CrossRef CAS PubMed; (b) Z. Li, N. Sharma, U. K. Sharma, J. Jacobs, L. Van Meervelt and E. V. Van der Eycken, Chem. Commun., 2016, 52, 5516 RSC.
- A. Kumar, Z. Li, S. K. Sharma, V. S. Parmar and E. V. Van der Eycken, Org. Lett., 2013, 15, 1874 CrossRef CAS PubMed.
- (a) S. G. Modha, A. Kumar, D. D. Vachhani, S. K. Sharma, V. S. Parmar and E. V. Van der Eycken, Chem. Commun., 2012, 48, 10916 RSC; (b) A. Kumar, D. D. Vachhani, S. G. Modha, S. K. Sharma, V. S. Parmar and E. V. Van der Eycken, Synthesis, 2013, 45, 2571 CrossRef CAS; (c) Z. Li, A. Kumar, S. K. Sharma, V. S. Parmar and E. V. Van der Eycken, Tetrahedron, 2015, 71, 3334 Search PubMed.
- (a) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS PubMed; (b) A. Daşan, A. Kulkarnia and B. Török, Green Chem., 2012, 14, 17 RSC; (c) E. Feng, Y. Zhou, F. Zhao, X. Chen, L. Zhang, H. Jiang and H. Liu, Green Chem., 2012, 14, 1888 RSC; (d) X. Zhang, Y. Zhou, H. Wang, D. Guo, D. Ye, Y. Xu, H. Jiang and H. Liu, Green Chem., 2011, 13, 397 RSC; (e) D. Ye, J. Wang, X. Zhang, Y. Zhou, X. Ding, E. Feng, H. Sun, G. Liu, H. Jiang and H. Liu, Green Chem., 2009, 11, 1201 RSC.
- (a) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (b) D. J. Ramón and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef PubMed; (c) B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed; (d) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC; (e) L. H. Choudhury and T. Parvin, Tetrahedron, 2011, 67, 8213 CrossRef CAS.
- Z. Li, A. Kumar, A. Peshkov and E. V. Van der Eycken, Tetrahedron Lett., 2016, 57, 754 CrossRef CAS.
- CCDC 1500316 6a contains the supplementary crystallographic data for this paper.†.
- (a) A. Klapars, S. Parris, K. W. Anderson and S. L. Buchwald, J. Am. Chem. Soc., 2004, 126, 3529 CrossRef CAS PubMed; (b) S. K. Chattopadhyay, S. Karmakar, T. Biswas, K. C. Majumdar, H. Rahaman and B. Roy, Tetrahedron, 2007, 63, 3919 CrossRef CAS.
- (a) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795 CrossRef PubMed; (b) R. Severin and S. Doye, Chem. Soc. Rev., 2007, 36, 1407 RSC; (c) J. M. Carney, P. J. Donoghue, W. M. Wuest, O. Wiest and P. Helquist, Org. Lett., 2008, 10, 3903 CrossRef CAS PubMed; (d) H. Wang, S. Jiao, K. Chen, X. Zhang, L. Zhao, D. Liu, Y. Zhou and H. Liu, Beilstein J. Org. Chem., 2015, 11, 416 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1500316. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra23180b |
| ‡ Both authors equally contributed. |
| This journal is © The Royal Society of Chemistry 2016 |