Highly-emissive solution-grown furan/phenylene co-oligomer single crystals†
Maxim S. Kazantsev *ab,
Ekaterina S. Frantsevaab,
Liudmila G. Kudriashovac,
Vladislav G. Konstantinovc,
Artur A. Mannanovcd,
Tatyana V. Rybalovaab,
Elena V. Karpovaab,
Inna K. Shundrinaab,
Gennadiy N. Kamaeve,
Maxim S. Pshenichnikovd,
Evgeny A. Mostovichab and
Dmitry Yu. Paraschuk*c
*ab,
Ekaterina S. Frantsevaab,
Liudmila G. Kudriashovac,
Vladislav G. Konstantinovc,
Artur A. Mannanovcd,
Tatyana V. Rybalovaab,
Elena V. Karpovaab,
Inna K. Shundrinaab,
Gennadiy N. Kamaeve,
Maxim S. Pshenichnikovd,
Evgeny A. Mostovichab and
Dmitry Yu. Paraschuk*c
aN. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Lavrentieva 9, Novosibirsk, 630090, Russian Federation. E-mail: maximkazantsev1988@gmail.com
bNovosibirsk State University, Pirogova 2, Novosibirsk, 630090, Russian Federation
cDepartment of Physics, International Laser Center, Lomonosov Moscow State University, Leninskie Gory 1/62, Moscow, 119991, Russian Federation. E-mail: paraschuk@gmail.com
dZernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, Groningen, 9747 AG, The Netherlands
eInstitute of Semiconductor Physics SB RAS, Lavrentieva 13, Novosibirsk, 630090, Russian Federation
First published on 22nd September 2016
Abstract
Solution-processed furan/phenylene co-oligomer single crystals combine high photoluminescence quantum yield (>65%) and efficient charge transport (mobility 0.12 cm2 V−1 s−1) making them promising materials for printable organic optoelectronics.
Materials combining high luminescence efficiency and efficient charge transport properties are in strong demand in organic optoelectronics for various light-emitting devices, e.g., light-emitting diodes and transistors, displays and electrically-pumped lasers.1–4 Attainment of both properties simultaneously presents a great challenge because high mobility requires close molecular packing that usually leads to weak emission.5 However, several exceptions from this trend have been found, e.g., thiophene/phenylene co-oligomers (TPCOs),3,6–8 distyrylbenzenes,1 anthracene derivatives9 and furan-containing molecules,10,11 for which a lucky combination of high fluorescence quantum yield (>50%) and efficient charge transport (mobility ≥ 0.1 cm2 V−1 s−1) has been reported. Furan incorporation is one of the most useful routes for increasing fluorescence resulting also in an additional benefit of increased solubility, which is highly favourable for industrial applications in solution-processed technologies.12–14 Whereas TPCO single crystals have shown their high potential for various light-emitting devices,2,3,6–8,15 analogous furan/phenylene co-oligomer single crystals have been far less studied.11 Moreover, up to date solution-grown organic semiconducting furan/phenylene single crystals have not yet been reported.
Here, we report on organic solution-processed semiconducting single crystals based on furan/phenylene co-oligomer 1,4-bis(5-phenylfuran-2-yl)benzene (BPFB) that combine high (>65%) photoluminescence quantum yield (PL QY) with efficient charge transport (mobility ∼ 0.1 cm2 V−1 s−1).
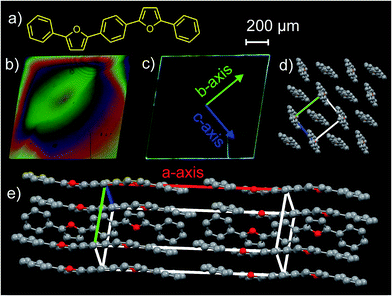
Fig. 1a demonstrates chemical structure of BPFB. It has an alternating furan/phenylene structural motif similar to well-studied 1,4-bis(5-phenylthiophene-2-yl)benzene (AC5).3,6,15 BPFB was synthesized by a Pd-catalyzed Suzuki cross-coupling reaction from 1,4-bis(5-bromofuran-2-yl)benzene and phenylboronic acid.
Thermogravimetric analysis and differential scanning calorimetry (Fig. S12†) indicate that BPFB is stable and does not show any signatures of decomposition in inert atmosphere so that only melting at 238 °C is observed followed by total sublimation. BPFB is also stable up to the melting temperature in oxidizing atmosphere (Fig. S12†). Cyclic voltammetry (CV) measurements demonstrate that the first oxidation peak is reversible indicating that BPFB is stable under multiple red/ox cycles that is highly favourable for charge transport (Fig. S13†). The highest occupied molecular orbital (HOMO) energy derived from the CV data was EHOMO = −5.26 eV, and the lowest unoccupied molecular orbital (LUMO) energy was calculated as ELUMO = EHOMO + Eg = −2.23 eV, where Eg = 3.03 eV is the optical gap corresponding to the absorption spectrum edge. The measured energies are in reasonable correspondence with the quantum chemistry calculations (ESI†).
The solubility of BPFB in toluene (ca. 1 g l−1) was sufficient for growing single crystals from solution by solvent–antisolvent crystallization.16 The crystals had a rhombus shape with lateral sizes of a few millimetres and thicknesses of several microns. Fig. 1b shows an optical micrograph of a BPFB crystal in transmitted light through crossed polarizers. In-plane rotation of the crystal results in a fully dark image (see Fig. S14†) as it should be for a single crystal with linear birefringence. The crystal image in UV light in Fig. 1c indicates that the PL escapes the crystal mainly at its edges and defects due to the waveguide effect. Atomic-force microscopy of BPFB single crystals (Fig. S14†) shows that their surface is flat with single molecular steps. Such a smooth surface for solution-grown crystals was observed earlier for TPCO single crystals grown by solvent–antisolvent crystallization.16
The X-ray diffraction experiments on BPFB single crystals (for details see ESI†) reveal that they are monoclinic and belong to P21/c space group. The unit cell parameters are as follows: a = 20.4117(19), b = 7.2937(8), c = 6.2000(5) Å, β = 97.731(3)°. The molecules are packed according to herringbone motif with the herringbone angle (angle between the average molecular planes) equal to 40.9° (Fig. 1d). The herringbone packing is controlled by C–H⋯π interactions (Table S1†), which compete with π–π interaction. The long molecular axis (connecting two carbons in p-positions of the terminal phenyls) is tilted by 87.8° against the main crystal plate (Fig. 1d and e).
To study semiconducting properties of BPFB single crystals, organic field-effect transistors (OFETs) were fabricated using the top-gate top-contact configuration with parylene N as a gate dielectric used earlier for TPCO crystals.8 In contrast to the more conventional bottom-gate top-contact device architecture used for TPCO3 and other crystals,9 our device configuration avoids tricky crystal lamination, and the OFET data are not affected by the crystal thickness. Fig. 2a demonstrates output current–voltage characteristics that correspond to p-type OFET behaviour with negligible contact effects. Fig. 2b presents transfer characteristics in the linear regime, which is considered to provide the most reliable mobility values.17 The hole mobility calculated in the linear regime 0.12 ± 0.01 cm2 V−1 s−1 was virtually the same as in the saturation regime (Fig. S19†). Fabricated series of five devices shows reproducible OFET characteristics (Table S3†). The measured charge mobility for BPFB single crystals is nearly two times higher than that reported for analogous TPCO single crystals (AC5) in unipolar devices3 and significantly higher than the mobility reported for thin film devices based on oligo(thienylfuran)s18 and oligofurans,19 and comparable with that reported for vapor-grown single crystals of longer furan/phenylene co-oligomers.11
 | ||
| Fig. 2 OFET data. (a) Output characteristics, the inset shows a crossed-polarized optical image of a crystal with painted source–drain contacts (before parylene and gate deposition); (b) transfer characteristics in the linear regime (transfer characteristics of this device in the saturation regime are presented in Fig. S19†); Vsd, and Vg are source–drain and gate voltages, respectively, μlin is the mobility extracted from the linear regime. | ||
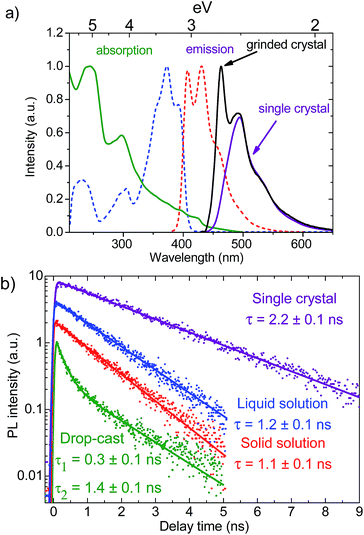
Optical properties of BPFB were studied in dilute liquid/solid solutions, a drop-cast sample, and single crystals. A liquid solution absorption spectrum in Fig. 3a shows a fine structure that is characteristic for furan-containing molecules12,13 as compared to other linear conjugated molecules such as oligothiophenes, oligo(phenylenevinylenes), oligo(thiophene-phenylenes) etc., which typically do not show the fine structure in solution. This fine structure could be assigned to higher rigidity of furan-containing conjugated oligomers.12,13 Another possible reason could be the molecule planarization through the intramolecular H⋯O interaction similarly to earlier reported for EDOT-type materials.20 The PL spectrum of BPFB demonstrates two maxima at 407 and 431 nm in liquid (Fig. 3a) and solid solutions (Fig. S23†).
The PL QY of BPFB in liquid solution is high, reaching 91 ± 3%. The BPFB PL QY is consistent with the high PL QY in 2,5-diarylfurans21 indicating that the phenyl-furan moiety favours efficient luminescence. One can speculate that the high PL QY is an important prerequisite for a highly luminescent crystalline phase. Indeed, the PL QY measured for BPFB single crystals is as high as 60% (Fig. S21b†). The PL spectra of BPFB single crystals are red-shifted by ∼0.45 eV (80 nm) as compared to that in solution (Fig. 3a). This shift can be assigned to PL reabsorption because of considerable overlap between the absorption and PL spectra (Fig. 3a) and to the solid state shift, i.e. to the higher polarizability of the molecular environment in the crystal as compared to solution. The latter can explain a ∼0.2 eV (30 nm) red-shift (the difference in the PL onset in solution and crystal) as follows from the PL data collected using a microscope (Fig. S25†), which were free of strong PL reabsorption as the PL passed in the crystal a path shorter than 10 μm.
To decrease the reabsorption effect on the PL QY, we grinded the crystal in small crystallites and measured the resulting PL spectrum.22 As follows from Fig. 3a, the intensity of the high energy part of the PL spectra (<500 nm) in the grinded sample is higher because of weaker PL reabsorption. We took into account the PL reabsorption from the integrated PL spectra of the as-grown and grinded crystals in Fig. S22.† As a result, the internal PL QY of BPFB single crystal is evaluated as 66%. This is a lower estimate for the internal PL QY as the 0–0 transition at 440 nm (Fig. S25†) in the PL spectra in the grinded sample (Fig. 3a) is still suppressed by PL reabsorption. Note that solution processing BPFB single crystals resulted in a nearly two times higher PL QY than that reported for AC5 single crystals grown by physical vapour transport.15
To further investigate the luminescent properties of BPFB, we measured the PL kinetics in the same samples, i.e. single crystals, dilute solid/liquid solutions, and drop-cast sample (Fig. 3b). As the kinetics in both liquid and solid solutions show similar lifetimes, we conclude that restriction of molecular motions in the solid solution does not have any influence the PL lifetime and spectra. However, the close packing of BPFB molecules in a single crystal results in a two-times longer PL lifetime. Note that PL in the solutions and crystal decays monoexponentially indicating the only luminophore type. On the other hand, PL in the drop-cast sample decays much faster and is not monoexponential (Fig. 3b) that can be assigned to disorder. From this we conclude that the structural order in the single crystal facilitates longer exciton lifetime.
The non-radiative recombination rate in organic crystals usually decreases upon cooling that is assigned to either less efficient exciton migration23 or slower non-radiative transitions,24 or both. We recorded the PL kinetic in the BPFB single crystal at 77 K (Fig. S24b†) and found that the PL lifetime is similar to the room temperature value. This indicates that the non-radiative recombination in BPFB single crystal is temperature independent. In contrast, at 77 K the time-resolved PL in the drop-cast sample becomes much longer than at room temperature (Fig. S24e†). This hints at a lower non-radiative recombination rate, which could be assigned to less mobile excitons. We speculate that the temperature-independent non-radiative recombination in BPFB single crystal is not due to exciton migration but mainly due to intersystem crossing, i.e., conversion of singlet excitons to triplet ones, which are about 1 eV lower in energy according to our gas-phase calculations (Fig. S18†).
Table 1 summarizes the photophysics data and shows that the radiative rate constant (kr) is more than two times lower in single crystal as compared to the solutions. At the same time, the PL QY decreases in the single-crystal only by 27% as compared to isolated BPFB molecules, i.e. in solution. As a result, the non-radiative rate constant (knr) increases in single crystal twice. The lower kr in crystal means that the transition dipole moment for closely packed BPFB molecules decreases that could be assigned to H-aggregation,25 which is indicative from the nearly collinear transition dipole moments oriented almost normally to the bc basal plane (Fig. S17†). The optical evidence of H-aggregation (i.e. a weak oscillator strength of the lowest energy optical transition) follows from weak absorption near the absorption edge in the absorption spectrum of the drop-cast sample in Fig. 3a. However, despite the lower transition dipole moment in BPFB aggregates, the well-ordered structure of single crystals still provides high PL QY (the very similar conclusion was reported for distyrylbenzene23), which is to the best of our knowledge the highest among furan co-oligomers and solution-grown semiconducting single crystals containing heteroaryls.
The low-defect structure of the BPFB single crystals was also confirmed by a much weaker PL spectral diffusion (i.e. temporal changes in the PL spectrum) in the single crystal as compared to the drop-cast sample (Fig. S24c and f†). Thus, all PL data suggest that the high luminescent properties of BPFB single crystals stem from their highly ordered structure.
We stress that no signatures of PL degradation were observed in BPFB single crystals after many repeated measurements of PL QY and kinetics on the very same samples neither during the measurements nor after a few weeks storing under ambient conditions. In contrast, PL in liquid and solid solutions degraded even after short exposure to light. These observations suggest that closely packed BPFB crystalline structure enhances the photostability and shelf lifetime.
In summary, furan/phenylene co-oligomer BPFB demonstrates the unique combination of high luminescence efficiency and efficient charge transport in a solution-processed single crystal. Taking into account suitable solubility and high thermal stability of BPFB, this combination opens the way for mass production of optoelectronic materials and devices using printing technologies.
Author contributions
MSK planned the experiments, grew the crystals and assembled the OFET samples. ESF and EAM synthesized the compound and performed quantum chemistry calculations. VGK measured the PL data of solid samples, and LGK processed them. AAM and MSP measured and analysed time-resolved PL data. TVR solved the crystal structure. EVK measured the PL data in solution. IKS performed the thermal analysis. GNK measured OFET data. MSK and DYP conceived and supervised the whole project. All the authors participated in the results discussions and manuscript writing.Acknowledgements
We thank V. V. Bruevich and O. V. Kozlov for useful discussions. The work on material synthesis, characterization and OFETs was supported by RFBR (project No. 16-33-60011 mol_a_dk). The work on steady-state optical studies was supported by Russian Science Foundation (project 15-12-30031). QY measurements were performed on the equipment purchased under the Lomonosov Moscow State University Program of Development. Authors would like to acknowledge the Multi-Access Chemical Service Center SB RAS and Siberian Supercomputing Center (http://www2.sscc.ru/).Notes and references
- J. Gierschner and S. Y. Park, J. Mater. Chem. C, 2013, 1, 5818–5832 RSC.
- H.-H. Fang, J. Yang, J. Feng, T. Yamao, S. Hotta and H.-B. Sun, Laser Photonics Rev., 2014, 8, 687–715 CrossRef.
- S. Hotta, T. Yamao, S. Z. Bisri, T. Takenobu and Y. Iwasa, J. Mater. Chem. C, 2014, 2, 965–980 RSC.
- J. Gierschner, S. Varghese and S. Y. Park, Adv. Opt. Mater., 2016, 2, 348–364 CrossRef.
- S. Varghese and S. Das, J. Phys. Chem. Lett., 2011, 2, 863–873 CrossRef CAS PubMed.
- S. Hotta and T. Yamao, J. Mater. Chem., 2011, 21, 1295–1304 RSC.
- T. Komori, H. Nakanotani, T. Yasuda and C. Adachi, J. Mater. Chem. C, 2014, 2, 4918–4921 RSC.
- L. G. Kudryashova, M. S. Kazantsev, V. A. Postnikov, V. V. Bruevich, Y. N. Luponosov, N. M. Surin, O. V. Borshchev, S. A. Ponomarenko, M. S. Pshenichnikov and D. Y. Paraschuk, ACS Appl. Mater. Interfaces, 2016, 8, 10088–10092 CAS.
- A. Dadvand, A. G. Moiseev, K. Sawabe, W.-H. Sun, B. Djukic, I. Chung, T. Takenobu, F. Rosei and D. F. Perepichka, Angew. Chem., Int. Ed., 2012, 51, 3837–3841 CrossRef CAS PubMed.
- K. Niimi, H. Mori, E. Miyazaki, I. Osaka, H. Kakizoe, K. Takimiya and C. Adachi, Chem. Commun., 2012, 48, 5892–5894 RSC.
- K. Oniwa, T. Kanagasekaran, T. Jin, M. Akhtaruzzaman, Y. Yamamoto, H. Tamura, I. Hamada, H. Shimotani, N. Asao, S. Ikeda and K. Tanigaki, J. Mater. Chem. C, 2013, 1, 4163–4170 RSC.
- O. Gidron and M. Bendikov, Angew. Chem., Int. Ed., 2014, 53, 2546–2555 CrossRef CAS PubMed.
- O. Gidron, N. Varsano, L. J. W. Shimon, G. Leitus and M. Bendikov, Chem. Commun., 2013, 49, 6256–6258 RSC.
- O. Gidron, A. Dadvand, W.-H. Sun, I. Chung, L. Shimon, M. Bendikov and D. Perepichka, J. Mater. Chem. C, 2013, 1, 4358–4367 RSC.
- Y. Yomogida, T. Takenobu, H. Shimotani, K. Sawabe, S. Z. Bisri, T. Yamao, S. Hotta and Y. Iwasa, Appl. Phys. Lett., 2010, 97, 173301 CrossRef.
- V. A. Postnikov, Y. I. Odarchenko, A. V. Iovlev, V. V. Bruevich, A. Y. Pereverzev, L. G. Kudryashova, V. V. Sobornov, L. Vidal, D. Chernyshov, Y. N. Luponosov, O. V. Borshchev, N. M. Surin, S. A. Ponomarenko, D. A. Ivanov and D. Y. Paraschuk, Cryst. Growth Des., 2014, 14, 1726–1737 CAS.
- V. Podzorov, MRS Bull., 2013, 38, 15–24 CrossRef.
- Y. Miyata, M. Terayama, T. Minari, T. Nishinaga, T. Nemoto, S. Isoda and K. Komatsu, Chem.–Asian J., 2007, 2, 1492–1504 CrossRef CAS PubMed.
- O. Gidron, A. Dadvand, Y. Sheynin, M. Bendikov and D. F. Perepichka, Chem. Commun., 2011, 47, 1976–1978 RSC.
- B. Milian-Medina, D. Wasserberg, S. C. J. Meskers, E. Mena-Osteritz, P. Bauerle and J. Gierschner, J. Phys. Chem. A, 2008, 112, 13282–13286 CrossRef CAS PubMed.
- F. Klukas, A. Grunwald, F. Menschel and T. J. J. Müller, Beilstein J. Org. Chem., 2014, 10, 672–679 CrossRef PubMed.
- R. Katoh, K. Suzuki, A. Furube, M. Kotani and K. Tokumaru, J. Phys. Chem. C, 2009, 113, 2961–2965 CAS.
- J. Gierschner, L. Luer, B. Milian-Medina, D. Oelkrug and H.-J. Egelhaaf, J. Phys. Chem. Lett., 2013, 4, 2686–2697 CrossRef CAS.
- S. Kanazawa, M. Ichikawa, T. Koyama and Y. Taniguchi, ChemPhysChem, 2006, 7, 1881–1884 CrossRef CAS PubMed.
- E. G. Mcrae and M. Kasha, J. Chem. Phys., 1958, 28, 721–722 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The detailed experimental procedures and instrumentation used in this research. CCDC 1478328. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra23160h |
| This journal is © The Royal Society of Chemistry 2016 |