4-Dialkylaminopyridine modified magnetic nanoparticles: as an efficient nano-organocatalyst for one-pot synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives in water†
Abstract
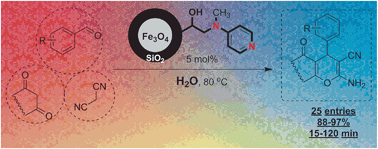
A novel heterogeneous magnetic nano-organocatalyst was developed using immobilization of 4-dialkylaminopyridine moieties on Fe3O4 magnetic nanoparticles (MNP–DMAP). It was synthesized using the reaction of MNP-oxiran (MNPO) with N-methylpyridin-4-amine. The MNPO substrate was produced via oxidation of vinyl groups on the surface of vinyl-functionalized MNP (VMNP) using H2O2. To synthesis VMNP, silica-coated magnetic nanoparticles (Fe3O4@SiO2) were reacted with trimethoxy(vinyl)silane. The MNP–DMAP catalyst shows remarkable activity in the synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives using a multicomponent reaction under mild conditions in water as a green solvent. The MNP–DMAP was reusable in this process for at least 10 times without any treatment in its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...