DOI:
10.1039/C6RA23119E
(Paper)
RSC Adv., 2016,
6, 104173-104182
Synthesis of ultralong NiSe nanobelts and their excellent adsorption properties towards malachite green in water
Received
16th September 2016
, Accepted 24th October 2016
First published on 24th October 2016
Abstract
NiSe ultralong nanobelts were synthesized by a solvothermal reaction of Ni with Se and KBH4 in ethylenediamine and subsequent annealing in Ar. The aspect ratios of NiSe ultralong nanobelts can be tuned by altering the reaction time. The adsorption performance of NiSe nanostructures with different aspect ratios was investigated, and the as-obtained NiSe ultralong nanobelts exhibited excellent adsorption capability of malachite green in wastewater, which could be attributed to their unique honeycomb-like assembly and high surface area. The adsorption process agreed well with the pseudo-second-order kinetics and the Langmuir isotherm equation. Thermodynamic parameters indicated that the adsorbent processes were spontaneous. Electrostatic attraction between malachite green and NiSe ultralong nanobelts was considered as the adsorption mechanism. The NiSe ultralong nanobelts could be regarded as a promising adsorbent for the removal of MG in wastewaters.
1. Introduction
In recent years, semiconductor nanocrystals with a defined size and shape have attracted considerable attention due to their size/shape-dependent physical and chemical properties and enormous potential as fundamental building blocks for nanoscale photoelectric devices.1–3 Nickel selenide (NiSe), an important p-type semiconductor with a desirable band gap of 2.0 eV, shows unique electronic and magnetic properties.4,5 To date, quantum dots,6 nanospheres,7,8 nanoparticles,9,10 nanowires,11,12 nanotubes,13,14 nanorod arrays,4 nano-dandelion arrays15 and sea urchin-like nanofiber assemblies16 of NiSe have been obtained. The NiSe quantum dots were used to fabricate fluorescent patterns and sensitized solar cells.6 The sea urchin-like NiSe nanofiber assemblies16 and nanowires12 displayed relatively high catalytic activity and durability for both oxygen evolution reaction and hydrogen evolution reaction. The NiSe nanotubes13 and nano-dandelion arrays15 were used as cathode materials for lithium-ion batteries, and showed superior lithium insertion–deinsertion properties. However, to the best of our knowledge, NiSe nanomaterials have never been reported to function as the adsorbents for organic dyes in water.
Malachite green (MG) selected in this study belongs to triphenylmethane dyes. It has been widely used in silk, wool, cotton, leather, paper and other industries.17 However, MG has highly toxic properties, which are known to cause diseases like eye burns, fast breathing, profuse sweating, and cancer at different parts of human body. Therefore, it is necessary to remove MG from wastewater before discharging to the environment. Activated carbon,18 zeolite,19 metallic oxide20,21 and graphite oxide22 have been used as adsorbents to remove MG from wastewater, and adsorption capacity was merely 263.58, 16.5, 142.08, 32.0 and 187.1 mg g−1, respectively. Therefore, the design and synthesis of efficient adsorbent is still a challenge. In addition, there has been no report on the morphology-dependent adsorption property for MG until now.
Herein, we report on the controlled synthesis of NiSe ultralong nanobelts, nanowires and nanorods by a solvothermal reaction of Ni with Se and KBH4 in ethylenediamine (En) and subsequent annealing in Ar. The aspect ratios of NiSe one-dimensional (1-D) nanostructures can be controlled by changing the solvothermal reaction time. The aspect ratio dependent adsorption property of these 1-D NiSe nanostructures for MG in water was investigated, and the adsorption mechanism was discussed in detail.
2. Experimental section
2.1 Sample preparation
All reagents used were of analytical purity and were used directly without further purification. Ethylenediamine (En), KBH4, and selenium powder were from the Sinopharm chemical reagent Co., Ltd. Nickel foils (99.99%) were from the Xi'an chemical reagent factory, China. In a typical procedure, 15 mL of En, a piece of nickel foil (1.3 × 2.5 cm2), 0.04 g (0.5 mmol) of selenium powder, and 0.04 g of KBH4 were added into a 30 mL of Teflon-lined autoclave. The autoclave was sealed, heated at 180 °C for 2–12 h. After the heat treatment, the autoclave was cooled to room temperature. The nickel foil was removed from the solution, rinsed with deionized water and absolute ethanol two times, respectively, and then was naturally dried, a black-colored layer was formed on the surface of the nickel foil. The as-prepared precursors were heated at 400 °C for 2 h in Ar, a black product was then formed on the nickel substrate surface.
2.2 Characterization
The morphology, dimension and crystal structures of the as-prepared products were analyzed using X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The XRD patterns were obtained on a Rigaku D/MAX2550 X-ray diffractometer with Cu Kα1 radiation (λ = 1.5406 Å). SEM analysis was performed by using a FEI Quanta 200 scanning electron microscope and a Hitachi SU8020 field emission scanning electron microscope (FESEM). TEM and electron diffraction images were obtained using a JEOL JEM-2100 transmission electron microscope at an accelerating voltage of 200 kV. The IR spectrum was recorded using a Bruker Equinx 55 Fourier transform IR spectrophotometer. The Brunauer–Emmett–Teller (BET) specific surface area determination was performed by N2 gas adsorption using an America Micromeritics ASAP 2020 surface analytical instrument.
2.3 Adsorption kinetic and equilibrium experiments
The adsorption kinetic experiments were carried out by adding 2.0 mg of the as-prepared 1-D NiSe nanostructures (adsorbent) to a 50 mL colorimetric tube filled with 50 mL of 23 mg L−1 of aqueous MG solution at room temperature. The aqueous samples were taken at pre-set time intervals. After removing the NiSe adsorbent by centrifugation, the MG concentration in the clear solution was measured using a Hitachi U-2910 spectrophotometer. The detection wavelength for MG was 616 nm. The amount of adsorption at time t, qt (mg g−1), was calculated by eqn (1):| |
 | (1) |
where C0 and Ct (mg L−1) are the MG concentration at the beginning of the experiment and at time t, respectively. V (L) the solution volume and W (g) are the mass of NiSe 1-D nanostructures used.
The adsorption equilibrium is achieved within 660 min for MG. The final concentration of MG remained in the solution was thus measured after 660 min. The amount of the dye at equilibrium qe (mg g−1) on the adsorbent samples was calculated from the following eqn (2):
| |
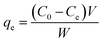 | (2) |
where
Ce (mg L
−1) is the MG concentration at the equilibrium states.
2.4 Determination of the Ni and Se ions released from the as-prepared NiSe samples
50 mL of 23 mg L−1 of aqueous MG solution was treated with 2.0 mg of the as-prepared ultralong NiSe nanobelts for 24 or 48 h, the NiSe adsorbents then were removed by centrifugation to obtain a clear solution. Transfer 5 mL of the clear solution to a 50 mL volumetric flask. Dilute to volume with 2% (v/v) HNO3 solution and mix well. Concentrations of Ni and Se ions in the solution were measured using inductively coupled plasma mass spectrometry (ICP-MS) (Thermo X series 2 ICP-MS, America).
3. Results and discussion
3.1 Structural and morphological characterization
3.1.1 SEM and XRD analysis. Fig. 1 shows SEM images and XRD patterns from the products synthesized in the Ni–Se–KBH4–En solvothermal reaction system at 180 °C for 12 h. The SEM images indicate that the samples consist of a large quantity of nanobelts with typical lengths in the range of several tens to several hundreds of micrometer. The numerous nanobelts self-assemble into honeycomb-like superstructures. Cross-sectional SEM image of a nanobelt shows a rectangle like cross section with a width of 48 nm, a thickness of 28 nm (the inset in Fig. 1b). The chemical compositions of the samples were measured by the EDS system attached to the SEM, and the result is shown in Fig. 1c. In the EDS spectrum C, N, Se and Ni elements are marked, and thus the precursor samples consist of C, N, Se, Ni and H elements. Hydrogen cannot be evidenced by EDS due to its low atomic number. Fig. 1d shows the XRD pattern of the precursor sample. The strong and sharp diffraction peaks reveal that it is well crystallized.
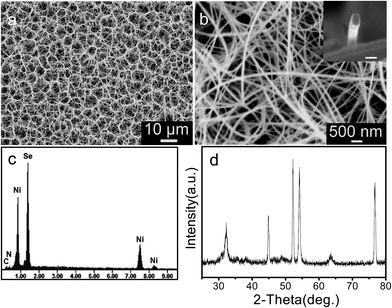 |
| | Fig. 1 (a, b) SEM images, (c) EDS spectra and (d) XRD pattern of the precursors synthesized at 180 °C for 12 h. | |
Fig. 2a–c displays the SEM images of the products obtained by annealing the NiSe(En)0.5 precursors at 400 °C for 2 h in Ar atmosphere. The SEM images reveal that after annealing the geometrical morphologies of the honeycomb-like micropatterns assembled from ultralong nanobelts are well preserved. The nanobelt showed in Fig. 2c has a rectangle like cross section with a typical width of 51 nm and a thickness of 20 nm. The corresponding XRD pattern is displayed in Fig. 2d. All diffraction peaks can be attributed to NiSe phase with a hexagonal structure, which are in a good agreement with Joint Committee on Powder Diffraction Standards (JCPDS) card no. 65-3425. In addition to the NiSe diffraction peaks, the metallic Ni diffraction peaks from the substrate are also observed.
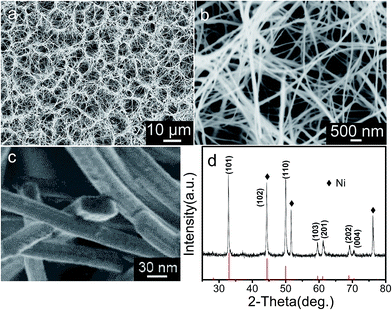 |
| | Fig. 2 (a–c) SEM images and (d) XRD pattern of the products obtained by annealing the precursors prepared at 180 °C for 12 h. The stick pattern is the standard XRD pattern for NiSe powders (JPCDS card no. 36-3425). | |
3.1.2 IR and TGA analysis. In order to confirm the chemical compositions of the precursors, IR spectrum and TGA of the precursor samples were measured, and the results are shown in Fig. 3. In the IR spectrum, the O–H characteristic vibration bands at 3436 and 1636 cm−1 may result from small quantity of adsorbed H2O on the samples.23,24 The bands at 1386, 2930, and 2856 cm−1 correspond to wagging, asymmetric and symmetric stretching vibrations of the –CH2,24 respectively. The C–N stretching vibration band appears at 1086 cm−1.25 The results suggest that there are En molecules in the precursors. According to ref. 26, the precursor is similar to ZnSe(En)0.5, may be NiSe(En)0.5. TGA results are given in Fig. 3b. The TGA curve shows that the precursors undergo a two-step weight-loss process. The first mass loss occurs at about 150 °C with a net weight loss of 4.1%, which is due to the removal of the adsorbed H2O on the samples.27 The second large weight loss at about 441 °C with a net weight loss of 19.0%, which is close to the expected value of 17.9% calculated for the change of NiSe(En)0.5 to NiSe. The results indicate that in the solvothermal system metallic Ni reacts with Se and En at 180 °C to form a NiSe–En hybrid compound, NiSe(En)0.5, then transformed into NiSe by calcining in an Ar gas atmosphere at 400 °C.
 |
| | Fig. 3 (a) IR spectrum and (b) TGA and DTG curves of the precursors synthesized at 180 °C for 12 h. | |
3.1.3 TEM analysis. Further structural details of the as-synthesized ultralong NiSe nanobelts were studied with TEM. Fig. 4a shows a typical TEM image of a single nanobelt. The NiSe nanobelt is uniform in width and thickness, and the width and thickness are 50 and 16 nm, respectively. Selected area electron diffraction (SAED) pattern and high-resolution TEM (HRTEM) image from the box in (a) are shown in Fig. 4b and c, respectively. The SAED is assigned to the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] zone axis of single-crystalline NiSe with a hexagonal structure (Fig. 4b). The interfringe distances measured from the HRTEM image are 0.26 and 0.19 nm, respectively, corresponding to the (001) and (110) lattice planes. The results confirm that the NiSe nanobelt is single-crystalline, grows along the [001] direction and is enclosed by (110), (
0] zone axis of single-crystalline NiSe with a hexagonal structure (Fig. 4b). The interfringe distances measured from the HRTEM image are 0.26 and 0.19 nm, respectively, corresponding to the (001) and (110) lattice planes. The results confirm that the NiSe nanobelt is single-crystalline, grows along the [001] direction and is enclosed by (110), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), (
0), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10) and (1
10) and (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) crystallographic facets. The schematic illustration of the crystal orientation is shown in Fig. 4d.
0) crystallographic facets. The schematic illustration of the crystal orientation is shown in Fig. 4d.
 |
| | Fig. 4 (a) Typical TEM image, (b) SAED pattern and (c) HRTEM image of an individual NiSe ultralong nanobelt, (d) the crystal orientation schematic illustration of the NiSe ultralong nanobelt. | |
3.1.4 Characterizations of NiSe samples with different aspect ratios. The aspect ratios of NiSe nanowires/nanobelts can be changed by tuning the solvothermal reaction time. The products obtained by annealing the precursors synthesized in the solvothermal system at 180 °C for 2, 6 and 12 h were analyzed with SEM (Fig. 5). The SEM observations reveal that nanorod arrays can be obtained by annealing the precursors synthesized at 180 °C for 2 h. The nanorods have lengths of 5–10 μm and a diameter of about 400 nm (Fig. 5a and b). The aspect ratio of the nanorods/nanowires increases with an increase on the solvothermal reaction time. As the solvothermal reaction time is 6 and 12 h, the length is in the range of 20–30 and 50–200 μm, respectively, as shown in Fig. 5c–f and Table 1. Fig. 6 shows the corresponding XRD patterns, which indicates that the 1-D nanostructures with various lengths are NiSe with the hexagonal structure.
 |
| | Fig. 5 SEM images of the products obtained by annealing the precursors prepared at 180 °C for 2 h (a, b), 6 h (c, d) and 12 h (e, f). | |
Table 1 The aspect ratio, BET specific surface area and adsorption capacity of the as-obtained NiSe nanorods, nanowires, ultralong nanobelts and octahedrons
| Adsorbent |
Nanorod |
Nanowire |
Ultralong nanobelt |
Octahedron |
| Solvothermal reaction time (h) |
2 |
6 |
12 |
12 |
| Aspect ratio |
13–25 |
200–300 |
1000–4000 |
— |
| BET specific surface area (m2 g−1) |
5.6 |
6.8 |
10.3 |
4.9 |
| Adsorption capacity (mg g−1) |
329.5 |
366.8 |
501.5 |
148 |
 |
| | Fig. 6 XRD patterns of the products obtained by annealing the precursors prepared at 180 °C for 2 h, 6 h and 12 h. | |
The nitrogen adsorption–desorption isotherm and the corresponding Barrett–Joyner–Halenda (BJH) pore-size distribution plot (inset) of the NiSe nanorods, nanowires and ultralong nanobelts are shown in Fig. 7. These hysteresis loops are ascribed to type H3 loops, indicating the presence of mesopores in three kinds of samples. The pore size distribution of the three kinds of samples peaked at ca. 60, 40 and 65 nm, respectively. These pores were formed by self-organization of NiSe nanorods, nanowires or ultralong nanobelts. The Brunauer–Emmett–Teller (BET) specific surface area of the NiSe nanorods, nanowires and ultralong nanobelts is measured to be 5.6, 6.8 and 10.3 m2 g−1, respectively, as shown in Table 1.
 |
| | Fig. 7 (a–c) The nitrogen adsorption–desorption isotherm of the as-obtained NiSe nanorods, nanowires and ultralong nanobelts, the insets show the corresponding BJH pore size distribution. | |
3.2 Adsorption kinetics
The NiSe nanorods, nanowires and ultralong nanobelts were used as adsorbents for MG removal. Fig. 8a shows the kinetics of adsorption on the three kinds of NiSe samples for an initial MG concentration of 23 mg L−1. Adsorption/desorption equilibriums between MG and the three kinds of samples can be achieved within 660 min. The adsorption capacities of the NiSe nanorods, nanowires and ultralong nanobelts for MG are 329.5, 366.8 and 501.5 mg g−1, respectively. Apparently, the adsorption capacity increases with an increase on the aspect ratios of the 1-D NiSe nanostructures, and adsorption capacity of NiSe ultralong nanobelts is the largest, as shown in Table 1.
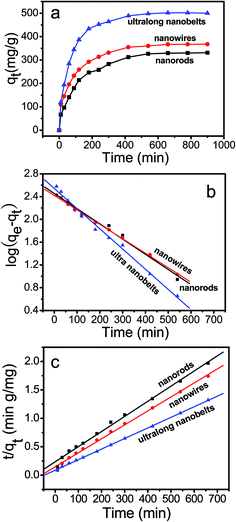 |
| | Fig. 8 (a) The variation of adsorption capacity with adsorption time for MG on the NiSe samples. (b, c) Pseudo-first-order and second-order kinetics for adsorption of MG onto the NiSe samples. | |
For comparison, all the adsorption capacities for MG reported in literature onto various adsorbents are collected and summarized in Table 2. It is found that the adsorption capacity of NiSe ultralong nanobelts for MG is higher than that of activated carbon,18 zeolite,19 NiO nanoarchitectures,20 graphite oxide22 and other adsorbents,21,29–38 only lower than porous C–ZrO2 composite.28 The comparative results demonstrated that the present ultralong NiSe nanobelts are excellent adsorbents for MG removal in wastewater.
Table 2 Adsorption capacity of MG onto all the adsorbents reported until now
| Adsorbent |
Dye |
Adsorption capacity (mg g−1) |
Data resource |
| Bamboo-based activated carbon |
MG |
263.58 |
18 |
| Natural zeolite |
MG |
16.5 |
19 |
| NiO nanoarchitectures |
MG |
142.08 |
20 |
| (Zn0.24Cu0.76)O particles |
MG |
32.0 |
21 |
| Graphite oxide |
MG |
187.1 |
22 |
| Porous C–ZrO2 composite |
MG |
2500 |
28 |
| Au-activated carbon |
MG |
172 |
29 |
| ZnO-activated carbon |
MG |
322.58 |
30 |
| Cd(OH)2-activated carbon |
MG |
19.0 |
31 |
| Clayey soil |
MG |
78.57 |
32 |
| Bentonite |
MG |
178.6 |
33 |
| Poly(acrylic acid)/SiO2 membranes |
MG |
220.49 |
34 |
| Oil palm trunk fibre |
MG |
149.35 |
35 |
| De-oiled soya |
MG |
12.88 |
36 |
| Ordered mesoporous carbons |
MG |
354.5 |
37 |
| Cellulose |
MG |
458.72 |
38 |
| NiSe ultralong nanobelts |
MG |
501.5 |
This study |
The kinetics of MG on the three kinds of NiSe samples was investigated by using two well-known pseudo-first-order and pseudo-second-order kinetic models. The pseudo-first-order equation is defined as:39,40
| |
 | (3) |
where
qe and
qt are the amounts of the dye adsorbed (mg g
−1) at equilibrium and at time
t (min), respectively, and
k1 (min
−1) is the pseudo-first-order rate constant.
Fig. 8b shows the adsorption data of MG fitted to the pseudo-first-order kinetic model, the
k1 values and the correlation coefficients (
R2) can be determined by linear regression. The obtained results are summarized in
Table 3.
Table 3 Pseudo-first-order adsorption kinetic constants of malachite green onto the three kinds of NiSe nanostructures
| Sample |
C0 (mg L−1) |
qe,exp (mg g−1) |
Pseudo-first-order model |
| qe,cal (mg g−1) |
K1 (×10−3 min−1) |
R2 |
S.D. (%) |
| Nanorod |
23 |
329.5 |
293.5 |
5.58 |
0.989 |
5.2 |
| Nanowire |
23 |
366.8 |
288.9 |
6.75 |
0.990 |
3.7 |
| Ultralong nanobelt |
23 |
501.5 |
367.6 |
8.93 |
0.991 |
3.5 |
The pseudo-second-order equation is expressed as follows:39
| |
 | (4) |
where
k2 (g mg
−1 min
−1) is the rate constant of second-order adsorption. The linear plot of
t/
qt versus t is shown in
Fig. 8c, the obtained values of
k2 and
R2 and kinetic parameters are summarized in
Table 4. As shown in
Tables 3 and
4, the normalized standard deviation (S.D.) and the
R2 values obtained by using the pseudo-second-order equation are better than that from the pseudo-first-order equation. The experimental
qe (
qe,exp) values are closer to the calculated
qe (
qe,cal) obtained by using the pseudo-second-order equation. These results suggest that the applicability of the pseudo-second-order model to describe the adsorption processes of MG onto the NiSe samples is feasible.
Table 4 Pseudo-second-order adsorption kinetic constants of malachite green onto the three kinds of NiSe nanostructures
| Sample |
C0 (mg L−1) |
qe,exp (mg g−1) |
Pseudo-second-order model |
| qe,cal (mg g−1) |
K2 (×10−5 g mg−1 min−1) |
R2 |
S.D. (%) |
| Nanorod |
23 |
329.5 |
367.9 |
3.12 |
0.992 |
4.5 |
| Nanowire |
23 |
366.8 |
399.8 |
4.55 |
0.996 |
3.6 |
| Ultralong nanobelt |
23 |
501.5 |
545.2 |
4.36 |
0.995 |
4.1 |
3.3 Adsorption isotherm
Fig. 9a shows the adsorption isotherms of MG onto the three kinds of NiSe samples. It was found that the adsorption capacities of NiSe samples for MG increased with increasing the dye concentration. The Langmuir and Freundlich isotherms were used to analyze the equilibrium adsorption data.
 |
| | Fig. 9 (a) Equilibrium adsorption capacity qe versus equilibrium concentrations Ce for the adsorption of MG onto the NiSe samples. (b, c) Adsorption isotherm of the adsorption of MG onto the NiSe samples: Langmuir model and Freundlich model. | |
The Langmuir adsorption is based on the assumption of monolayer adsorption on a homogenous adsorbent surface, and all of the adsorption sites being equivalent.10 The Langmuir equation is expressed as:38
| |
 | (5) |
where
qm is the Langmuir monolayer adsorption capacity of adsorbent (mg g
−1), and
KL is Langmuir constant. The parameters
qm and
KL were determined from the slope and intercept of the linear plot of 1/
qe versus 1/
Ce (
Fig. 9b), and the values of
qm,
KL and the correlation coefficients (
R2) are tabulated in
Table 5.
Table 5 Isotherm parameters of Malachite green onto the three kinds of NiSe samples
| Sample |
Langmuir isotherm |
Freundlich isotherm |
| qmax (mg g−1) |
KL (L mg−1) |
R2 |
KF (mg g−1) |
1/n |
R2 |
| Nanorod |
446.8 |
0.2695 |
0.9851 |
123.7 |
0.4436 |
0.9800 |
| Nanowire |
491.5 |
0.3206 |
0.9971 |
146.4 |
0.4357 |
0.9906 |
| Ultralong nanobelt |
683.2 |
0.5152 |
0.9931 |
334.0 |
0.4284 |
0.9481 |
Freundlich isotherm is an empirical model assuming that the adsorption process takes place on heterogeneous surfaces composed of different classes of adsorption sites. The Freundlich equation is expressed as:36,38
| |
 | (6) |
where
KF is Freundlich constants related to adsorption capacity, and 1/
n is the heterogeneity factor reflecting the adsorption intensity. The constants
KF and
n can be determined by the intercept and the slope of the linear plot of log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus
qe versus log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce (
Fig. 9c). The calculated values of
KF,
n and the correlation coefficient (
R2) were given in
Table 5. As shown in
Table 5, all the values of
R2 obtained from the Langmuir model are higher than that from Freundlich, indicating that MG are adsorbed on the surface of NiSe samples in monolayer coverage. The monolayer adsorption capacity of three kinds of NiSe samples determined from the Langmuir isotherm is 683.2, 491.5 and 446.8 mg g
−1 for MG adsorption, respectively.
3.4 Adsorption thermodynamics
The qe and Ce of the ultralong NiSe nanobelts for MG were measured at 303, 313 and 323 K. Three basic thermodynamic parameters, Gibbs energy change (ΔG°), enthalpy change (ΔH°) and entropy change (ΔS°) were calculated using following eqn (7)–(9).33,38| |
 | (7) |
| |
 | (8) |
where ΔG° is the change in Gibbs energy (kJ mol−1), ΔH° is the change in enthalpy (kJ mol−1), and ΔS° is the entropy change (J mol−1 K−1), KC is the distribution coefficient of adsorption, T is the adsorption temperature (K), and R is the ideal gas constant (8.314 J mol−1 K−1). ΔH° and ΔS° were calculated from the slope and intercept of the linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KC versus 1/T. The as-obtained thermodynamics parameters are shown in Table 6. The negative value of ΔG° indicates that the adsorption of MG on adsorbents is spontaneous and favorable process. The numerical values of ΔG° were in the range from −20 to 0 (kJ mol−1), which indicated the mechanism physical adsorption depending on electrostatic interaction.38 The positive value of ΔH° indicates that adsorption of MG onto the ultralong NiSe nanobelts is an endothermic process. Moreover, the positive value of ΔS° suggests good affinity of MG towards the adsorbents and increases randomness at the solid–solution interface. This is the normal consequence of the physical adsorption phenomenon and shows that adsorption takes place through electrostatic attraction.31
KC versus 1/T. The as-obtained thermodynamics parameters are shown in Table 6. The negative value of ΔG° indicates that the adsorption of MG on adsorbents is spontaneous and favorable process. The numerical values of ΔG° were in the range from −20 to 0 (kJ mol−1), which indicated the mechanism physical adsorption depending on electrostatic interaction.38 The positive value of ΔH° indicates that adsorption of MG onto the ultralong NiSe nanobelts is an endothermic process. Moreover, the positive value of ΔS° suggests good affinity of MG towards the adsorbents and increases randomness at the solid–solution interface. This is the normal consequence of the physical adsorption phenomenon and shows that adsorption takes place through electrostatic attraction.31
Table 6 Thermodynamic parameters for the adsorption of MG onto the NiSe ultralong nanobelts
| ΔH° (kJ mol−1) |
ΔS° (J mol−1) |
ΔG° (kJ mol−1) |
| 303 K |
313 K |
323 K |
| 40.71 |
167.39 |
−10.252 |
−11.225 |
−13.653 |
3.5 Adsorption mechanism
As shown in Table 1, the specific surface area of the 1-D NiSe nanostructures increased with an increase on the aspect ratios, and the adsorption performance was thus enhanced. The high specific surface area could provide more reactive adsorption sites for the adsorption reactions, which resulted in high adsorption performance.41 Moreover, NiSe octahedrons were prepared by a solvothermal reaction of NiCl2·6H2O with Se and KBH4 in pyridine at 180 °C for 12 h. Fig. 10a–c shows SEM images and XRD pattern of the NiSe octahedrons with the sizes of 300 to 420 nm and the hexagonal structure. The NiSe octahedrons were used to adsorb MG in water. The adsorption capacity on NiSe octahedrons was measured to be 148 mg g−1, it is dramatically lower than that of ultralong NiSe nanobelts, as shown in Fig. 10d and Table 1. Fig. 11a shows the nitrogen adsorption–desorption isotherm and the corresponding BJH pore-size distribution plot (inset) of the NiSe octahedrons, which indicated that the NiSe samples have mesopores with a peak pore at about 50 nm. The BET specific surface area of the NiSe octahedrons was measured to be 4.9 m2 g−1, and less than that of the ultralong NiSe nanobelts, as seen in Table 1. The results reveal that the excellent adsorption capability of the NiSe ultralong nanobelts for MG can be attributed to their unique honeycomb-like assembly and high surface area.
 |
| | Fig. 10 (a–c) SEM images and XRD pattern of the octahedrons. The stick pattern is the standard XRD pattern for NiSe powders (JPCDS card no. 65-3425). (d) The variation of adsorption capacity with adsorption time for MG on the NiSe nanobelts and NiSe octahedrons. | |
 |
| | Fig. 11 (a) The nitrogen adsorption–desorption isotherm of the as-obtained NiSe octahedrons, the insets show the corresponding BJH pore size distribution. (b) Zeta potentials of NiSe ultralong nanobelts as a function pH value. | |
The point of zero charge (pHPZC) of the NiSe ultralong nanobelts was determined by a potentiometric method.42 As shown in Fig. 11b, the pHPZC was estimated to be about 8.7. In the neutral MG solution, the NiSe samples have a positive surface charge. MG is a cationic dye, and its molecule structure and formula are shown in Fig. 12a. There are HC2O4− and C23H25N2+ ions in the MG molecules. We thus conclude that the interaction between the NiSe samples and MG should be electrostatic attraction. The positive surface charge of NiSe samples absorbed first HC2O4− to form a negatively charged layer, and then positive charged C23H25N2+ ions were adsorbed. The most likely structure is described in Fig. 12b. Thus, the electrostatic attraction between malachite green and NiSe ultralong nanobelts was considered as the main adsorption mechanism.
 |
| | Fig. 12 (a) The molecule structure and formula of MG, (b) the most likely structure of MG onto the surface of the NiSe nanobelt. | |
To evaluate the safety of the ultralong NiSe nanobelts for removing of MG in water, the concentrations of Ni and Se ions released from the NiSe ultralong nanobelts were measured using ICP-MS, and the results are listed in Table 7. It is clear that the concentrations of Ni and Se ions are less than the maximum acceptable concentrations of Ni and Se ions in drinking water recommended the World Health Organization (WHO)43,44 and Health Ministry of China (HMC),45 which indicated that the prepared NiSe nanobelts should be safe for the aim of application in the removal of MG.
Table 7 The concentrations of Ni ion and Se ions in the MG solution after being treated with the NiSe ultralong nanobelts for 24 h and 48 h
| Analyte |
Determination value (g L−1) |
Permissible limit (g L−1) |
| 24 h |
48 h |
WHO |
HMC |
| Ni ion |
6.83 × 10−7 |
9.46 × 10−7 |
1.0 × 10−4 |
2.0 × 10−5 |
| Se ion |
1.23 × 10−6 |
3.79 × 10−6 |
4.0 × 10−5 |
1.0 × 10−5 |
4. Conclusions
In summary, 1-D NiSe nanostructures have been synthesized by a solvothermal reaction of Ni with Se and KBH4 in En and subsequent annealing in Ar. The aspect ratios of 1-D NiSe nanostructures can be changed by tuning the solvothermal reaction time. Adsorption property of the 1-D NiSe nanostructure for MG is increased with increasing the aspect ratios. The kinetics adsorption is a pseudo-second order process. The equilibrium data fitted well with the Langmuir model. The thermodynamic study indicated that the adsorption of MG onto NiSe samples was spontaneous and an endothermic process. Electrostatic attraction between malachite green and NiSe ultralong nanobelts was considered as the main adsorption mechanism. The as-obtained NiSe ultralong nanobelt exhibits excellent adsorption capability with a maximum capacity of 501.5 mg g−1, which is expected to be a promising adsorbent for the removal of MG pollutants from wastewater.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21073116 and 21501116), and the Fundamental Research Funds for the Central Universities (GK201601003).
Notes and references
- A. I. Hochbaum and P. D Yang, Chem. Rev., 2010, 110, 527–546 CrossRef CAS PubMed.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- B. Z. Tian, T. J. Kempa and C. M. Lieber, Chem. Soc. Rev., 2009, 38, 16–24 RSC.
- X. Yu, W. D. Sun and Y. Chu, New J. Chem., 2014, 38, 70–76 RSC.
- M. Salavati-Niasari and A. Sobhani, Opt. Mater., 2013, 35, 904–909 CrossRef CAS.
- L. Ling, L. Zhu, Q. Zhang, C. F. Wang and S. Chen, J. Mater. Chem. C, 2015, 3, 473–478 RSC.
- I. T. Sines and R. E. Schaak, J. Am. Chem. Soc., 2011, 133, 1294–1297 CrossRef CAS PubMed.
- W. D. Shi, X. Zhang and G. B. Che, Int. J. Hydrogen Energy, 2013, 38, 7037–7045 CrossRef CAS.
- P. P. George, V. G. Pol, Y. Koltypin, J. M. Calderon-Moreno, I. Genish, M. S. Ben-David and A. Gedanken, RSC Adv., 2012, 2, 11725–11730 RSC.
- W. Zhang and R. Xu, Int. J. Hydrogen Energy, 2012, 37, 17899–17909 CrossRef CAS.
- Z. B. Zhuang, Q. Peng, J. Zhuang, X. Wang and Y. D. Li, Chem.–Eur. J., 2006, 12, 211–217 CrossRef CAS PubMed.
- C. Tang, N. Y. Cheng, Z. H. Pu, W. Xing and X. P. Sun, Angew. Chem., Int. Ed., 2015, 54, 9351–9355 CrossRef CAS PubMed.
- L. W. Mi, H. Sun, Q. Ding, W. H. Chen, C. T. Liu, H. W. Hou, Z. Zheng and C. Y. Shen, Dalton Trans., 2012, 12595–12600 RSC.
- Y. Z. Piao, J. Electrochem. Sci. Technol., 2012, 3, 35–43 CrossRef CAS.
- L. W. Mi, Q. Ding, H. Sun, W. H. Chen, Y. Y. Zhang, C. T. Liu, H. W. Hou, Z. Zheng and C. Y. Shen, CrystEngComm, 2013, 15, 2624–2630 RSC.
- M. R. Gao, Z. Y. Lin, T. T. Zhuang, J. Jiang, Y. F. Xu, Y. R. Zheng and S. H. Yu, J. Mater. Chem., 2012, 22, 13662–13668 RSC.
- W. Xu, Z. F. Deng and G. M. Li, Ind. Eng. Chem. Res., 2012, 51, 16188–16195 CrossRef CAS.
- B. H. Hameed and M. I. El-Khaiary, J. Hazard. Mater., 2008, 157, 344–351 CrossRef CAS PubMed.
- R. P. Han, Y. Wang, Q. Sun, L. L. Wang, J. Y. Song, X. T. He and C. C. Dou, J. Hazard. Mater., 2010, 175, 1056–1061 CrossRef CAS PubMed.
- A. H. Wei, B. Liu, H. Zhao, Y. Chen, W. L. Wang, Y. Ma, H. Q. Yang and S. Z. Liu, Chem. Eng. J., 2014, 239, 141–148 CrossRef CAS.
- N. M. Jacob, P. Kuruva, G. Madras and T. Thomas, Ind. Eng. Chem. Res., 2013, 52, 16384–16395 CrossRef.
- P. Bradder, S. K. Ling, S. B. Wang and S. M. Liu, J. Chem. Eng. Data, 2011, 56, 138–141 CrossRef CAS.
- M. H. Chen and L. Gao, Mater. Chem. Phys., 2005, 91, 437–441 CrossRef CAS.
- Q. Li, Y. J. Chen, T. Yang, D. N. Lei, G. H. Zhang, L. Mei, L. B. Chen, Q. H. Li and T. H. Wang, Electrochim. Acta, 2013, 90, 80–89 CrossRef CAS.
- L. H Zhang, H. Q. Yang, J. Yu, F. H. Shao, L. Li, F. H. Zhang and H. Zhao, J. Phys. Chem. C, 2009, 113, 5434–5443 Search PubMed.
- L. H. Zhang, H. Q. Yang, L. Li, R. G. Zhang, R. N. Liu, J. H. Ma, X. L. Xie and F. Gao, Inorg. Chem., 2008, 47, 11950–11957 CrossRef CAS PubMed.
- Y. Y. Yang, Y. R. Liang, Z. Y. Zhang, Y. D. Zhang, H. Y. Wu and Z. A. Hu, J. Alloys Compd., 2016, 658, 621–628 CrossRef CAS.
- P. A. Deshpande, S. Polisetti and G. Madras, Langmuir, 2011, 27, 3578–3587 CrossRef CAS PubMed.
- M. Roosta, M. Ghaedi, N. Shokri, A. Daneshfar, R. Sahraei and A. Asghari, Spectrochim. Acta, Part A, 2014, 118, 55–65 CrossRef CAS PubMed.
- M. Ghaedi, A. Ansari, M. H. Habibi and A. R. Asghari, J. Ind. Eng. Chem., 2014, 20, 17–28 CrossRef CAS.
- M. Ghaedi and N. Mosallanejad, J. Ind. Eng. Chem., 2014, 20, 1085–1096 CrossRef CAS.
- P. Saha, S. Chowdhury, S. Gupta and I. Kumar, Chem. Eng. J., 2010, 165, 874–882 CrossRef CAS.
- E. Bulut, M. Ozacar and I. A. Sengil, Microporous Mesoporous Mater., 2008, 115, 234–246 CrossRef CAS.
- R. Xu, M. Jia, Y. L. Zhang and F. T. Li, Microporous Mesoporous Mater., 2012, 149, 111–118 CrossRef CAS.
- B. H. Hameed and M. I. El-Khaiary, J. Hazard. Mater., 2008, 154, 237–244 CrossRef CAS PubMed.
- A. Mittal, L. Krishnan and V. K. Gupta, Sep. Purif. Technol., 2005, 43, 125–133 CrossRef CAS.
- Y. Tian, P. Liu, X. F. Wang and H. S. Lin, Chem. Eng. J., 2011, 171, 1263–1269 CrossRef CAS.
- Y. M. Zhou, M. Zhang, X. Y. Hu, X. H. Wang, J. Y. Niu and T. S. Ma, J. Chem. Eng. Data, 2013, 58, 413–421 CrossRef CAS.
- I. A. W. Tan, A. L. Ahmad and B. H. Hameed, J. Hazard. Mater., 2009, 164, 473–482 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Chem. Eng. J., 1998, 70, 115–124 CrossRef CAS.
- B. Cheng, Y. Le, W. Q. Cai and J. G. Yu, J. Hazard. Mater., 2011, 185, 889–897 CrossRef CAS PubMed.
- C. J. Pei, G. P. Han, Y. Zhao, H. Zhao, B. Liu, L. J. Cheng, H. Q. Yang and S. Z. Liu, J. Hazard. Mater., 2016, 318, 732–741 CrossRef CAS PubMed.
- A. B. Holmesa and F. X. Gu, Environ. Sci.: Nano, 2016, 3, 982–996 RSC.
- H. N. M. E. Mahmud, A. K. O. Huq and R. B. Yahy, RSC Adv., 2016, 6, 14778–14791 RSC.
- National Standards of the People's Republic of China, Standards for Drinking Water Quality (GB5749-2006).
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 
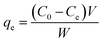

![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] zone axis of single-crystalline NiSe with a hexagonal structure (Fig. 4b). The interfringe distances measured from the HRTEM image are 0.26 and 0.19 nm, respectively, corresponding to the (001) and (110) lattice planes. The results confirm that the NiSe nanobelt is single-crystalline, grows along the [001] direction and is enclosed by (110), (
0] zone axis of single-crystalline NiSe with a hexagonal structure (Fig. 4b). The interfringe distances measured from the HRTEM image are 0.26 and 0.19 nm, respectively, corresponding to the (001) and (110) lattice planes. The results confirm that the NiSe nanobelt is single-crystalline, grows along the [001] direction and is enclosed by (110), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), (
0), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10) and (1
10) and (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) crystallographic facets. The schematic illustration of the crystal orientation is shown in Fig. 4d.
0) crystallographic facets. The schematic illustration of the crystal orientation is shown in Fig. 4d.







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus log
qe versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce (Fig. 9c). The calculated values of KF, n and the correlation coefficient (R2) were given in Table 5. As shown in Table 5, all the values of R2 obtained from the Langmuir model are higher than that from Freundlich, indicating that MG are adsorbed on the surface of NiSe samples in monolayer coverage. The monolayer adsorption capacity of three kinds of NiSe samples determined from the Langmuir isotherm is 683.2, 491.5 and 446.8 mg g−1 for MG adsorption, respectively.
Ce (Fig. 9c). The calculated values of KF, n and the correlation coefficient (R2) were given in Table 5. As shown in Table 5, all the values of R2 obtained from the Langmuir model are higher than that from Freundlich, indicating that MG are adsorbed on the surface of NiSe samples in monolayer coverage. The monolayer adsorption capacity of three kinds of NiSe samples determined from the Langmuir isotherm is 683.2, 491.5 and 446.8 mg g−1 for MG adsorption, respectively.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KC versus 1/T. The as-obtained thermodynamics parameters are shown in Table 6. The negative value of ΔG° indicates that the adsorption of MG on adsorbents is spontaneous and favorable process. The numerical values of ΔG° were in the range from −20 to 0 (kJ mol−1), which indicated the mechanism physical adsorption depending on electrostatic interaction.38 The positive value of ΔH° indicates that adsorption of MG onto the ultralong NiSe nanobelts is an endothermic process. Moreover, the positive value of ΔS° suggests good affinity of MG towards the adsorbents and increases randomness at the solid–solution interface. This is the normal consequence of the physical adsorption phenomenon and shows that adsorption takes place through electrostatic attraction.31
KC versus 1/T. The as-obtained thermodynamics parameters are shown in Table 6. The negative value of ΔG° indicates that the adsorption of MG on adsorbents is spontaneous and favorable process. The numerical values of ΔG° were in the range from −20 to 0 (kJ mol−1), which indicated the mechanism physical adsorption depending on electrostatic interaction.38 The positive value of ΔH° indicates that adsorption of MG onto the ultralong NiSe nanobelts is an endothermic process. Moreover, the positive value of ΔS° suggests good affinity of MG towards the adsorbents and increases randomness at the solid–solution interface. This is the normal consequence of the physical adsorption phenomenon and shows that adsorption takes place through electrostatic attraction.31








