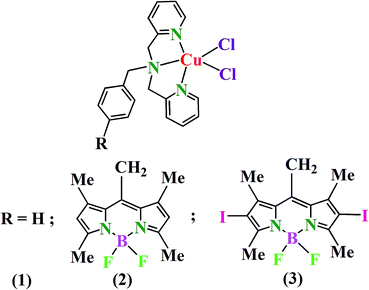
BODIPY appended copper(II) complexes for cellular imaging and singlet oxygen mediated anticancer activity in visible light†
Arnab Bhattacharyyaa,
Akanksha Dixitb,
Samya Banerjeea,
Bijan Roy a,
Arun Kumara,
Anjali A. Karande*b and
Akhil R. Chakravarty*a
a,
Arun Kumara,
Anjali A. Karande*b and
Akhil R. Chakravarty*a
aDepartment of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560012, India. E-mail: arc@ipc.iisc.ernet.in; Tel: +91-80-22932533
bDepartment of Biochemistry, Indian Institute of Science, Bangalore 560012, India. E-mail: anjali@biochem.iisc.ernet.in; Tel: +91-80-22932306
First published on 20th October 2016
Abstract
Copper(II) complexes of N,N,N-donor dipicolylamine ligands, viz. [Cu(L1)Cl2] (1), [Cu(L2)Cl2] (2) and [Cu(L3)Cl2] (3) with L2 and L3 having BODIPY (borondipyrromethene) moieties were synthesized, characterized and their photocytotoxicity studied. Complex 1 was structurally characterized by X-ray crystallography. It has copper(II) in a distorted square-pyramidal geometry (τ = 0.11) with two chloride ligands in axial-equatorial cis-disposition. The one-electron paramagnetic and redox active complexes are non-electrolytic in DMF. They behave as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytes in 1
2 electrolytes in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (v/v) DMF–H2O. The BODIPY complexes 2 and 3 show respective visible bands at 501 nm and 535 nm in DMF. Complex 2 displays an emission band at 515 nm in DMF (λex = 465 nm) with a quantum yield (ΦF) value of 0.14. This complex, as a fluorogenic probe for cellular imaging, shows cytosolic localisation of the complex in HeLa and MCF-7 cancer cells. Complex 3 having a diiodo BODIPY unit in L3 is non-emissive but acts as an efficient photosensitizer showing a significant PDT effect with apoptotic cell death forming singlet oxygen as ROS (ϕΔ = 0.53) and giving respective IC50 values of 0.15 ± 0.02 and 0.17 ± 0.03 μM in HeLa and MCF-7 cancer cells in visible light of 400–700 nm, while being less toxic in the dark.
9 (v/v) DMF–H2O. The BODIPY complexes 2 and 3 show respective visible bands at 501 nm and 535 nm in DMF. Complex 2 displays an emission band at 515 nm in DMF (λex = 465 nm) with a quantum yield (ΦF) value of 0.14. This complex, as a fluorogenic probe for cellular imaging, shows cytosolic localisation of the complex in HeLa and MCF-7 cancer cells. Complex 3 having a diiodo BODIPY unit in L3 is non-emissive but acts as an efficient photosensitizer showing a significant PDT effect with apoptotic cell death forming singlet oxygen as ROS (ϕΔ = 0.53) and giving respective IC50 values of 0.15 ± 0.02 and 0.17 ± 0.03 μM in HeLa and MCF-7 cancer cells in visible light of 400–700 nm, while being less toxic in the dark.
Introduction
Photodynamic therapy (PDT) has emerged as a non-invasive therapeutic modality to treat cancer.1–5 PDT causes selective damage only to the photoexposed cancer cells thus not affecting the unexposed healthy cells. The primary requirements of PDT are a photosensitizer, molecular oxygen and a light source. The PDT drug Photofrin upon red light activation generates singlet oxygen (1O2) as the reactive oxygen species (ROS) via a type-II pathway.6 Photofrin is known to localize in the mitochondria of the cells.7 Mitochondrial localization circumvents the drawbacks associated with the nuclear targeting chemotherapeutic drugs like cisplatin.8,9 Photofrin, however, suffers from two major drawbacks: (i) skin photosensitivity for a long period and (ii) oxidative degradation of haemoglobin leading to formation of bilirubin causing jaundice.10,11 Recent reports have shown that transition metal complexes can be suitably designed for efficient and targeted PDT activity.12–20 The present work stems from our interest to incorporate 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) dyes as photosensitizers into the ligand framework for synthesis of the metal complexes.21–25 BODIPY dyes were studied as potent PDT agents due their singlet oxygen generation ability and for their novel spectroscopic properties.26–30 The dyes alone in general show poor aqueous solubility. Binding to a metal ion could enhance their bioavailability. Besides, BODIPY dyes can be suitably modified and their fluorescence and photosensitizing abilities can be tuned by simple chemical modifications.26,27 For example, with heavy atom substitution in the central indacene core, one can convert it to an excellent photosensitizer with enhanced ability to generate singlet oxygen.28 In addition, due to their inherent emissive nature, BODIPY dyes without heavy atom are suitable for cellular imaging. Although there are several reports of using BODIPY dyes as potential PDT agents, the virtually unexplored coordination complexes having BODIPY as a photosensitizer could make a significant advancement in PDT.21–25,31–35Herein, we present copper(II) complexes, viz. [Cu(L1)Cl2] (1), [Cu(L2)Cl2] (2) and [Cu(L3)Cl2] (3), where L2 and L3 are dipicolylamine based non-iodinated and iodinated BODIPY ligands and L1 is benzyldipicolylamine (Fig. 1, Schemes S1 and S2, ESI†). Complex 1 is used as a control to study the role of the BODIPY moieties in 2 and 3 in showing any PDT activity. Complex 1 with a similar core structure as that of 2 and 3 is structurally characterized by X-ray crystallography. Copper as a bioessential metal ion is chosen for its affinity to form stable complexes with the N,N,N-donor ligands thus preventing any leaching of this metal into the biological medium. Complex 2 having the emissive BODIPY ligand L2 is used for cellular imaging. Complex 3 having the diiodo BODIPY ligand L3 is used as a photosensitizer and the complex displayed remarkable photocytotoxic effect in HeLa (human cervical carcinoma) and MCF-7 (human breast adenocarcinoma) cancer cells giving IC50 values in the nanomolar range while remaining less toxic in the dark.
Results and discussion
Synthesis and general aspects
Complexes 1–3 were synthesized in good yields by reacting CuCl2·2H2O with respective dipicolylamine ligands in anhydrous methanol and acetonitrile. The complexes were characterized from the elemental analysis and spectral data. The selected physicochemical data are presented in Table 1. The ESI-MS spectra in MeOH gave essentially a single peak corresponding to [M − Cl]+ [Fig. S1–S3, ESI†]. The mass spectral data indicate rapid loss of one chloride ligand. The UV-visible spectra in DMF showed bands assignable to the intraligand transition of the BODIPY ligands (Fig. 2(a)). Complex 1 displayed a band at 246 nm. Complexes 2 and 3 showed respective intense band at 501 and 535 nm involving the BODIPY moiety (Fig. 2(a) and S4, ESI†).36 The metal centred weak d–d bands were observed for the complexes within 600–800 nm. The absorption maximum for complex 1 was located at 747 nm which is blue shifted to 677 nm and 676 nm for complexes 2 and 3 respectively (Fig. S6, ESI†). Emissive properties of the complexes were investigated in DMF. Complex 1 in absence of any fluorophoric moiety did not show any emissive property. Complex 2 having fluorescent BODIPY moiety showed an emission maximum at 515 nm when excited at 465 nm with a fluorescence quantum yield (ΦF) of 0.14. Complex 3 having photosensitizer BODIPY moiety showed emission maximum at 560 nm (λex = 510 nm) with a very low quantum yield value of 0.01 (Fig. 2(b)). A significant decrease in the fluorescence quantum yield of the di-iodo BODIPY appended Cu(II) complex provides an indirect evidence of its better photosensitizing ability compared to the non-iodinated counterpart. The FT-IR spectra in solid state displayed characteristic aromatic C–H stretching vibrations near 3000 cm−1 and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations between 1400–1500 cm−1 for all the complexes. The strong band near 1610 cm−1 is assignable to the ν(C
C stretching vibrations between 1400–1500 cm−1 for all the complexes. The strong band near 1610 cm−1 is assignable to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of the pyridine rings of the dipicolylamine ligands (Fig. S7–S9, ESI†). The complexes are redox active showing cyclic voltammograms in DMF-0.1 M [nBu4N](ClO4) for the Cu(II)/Cu(I) redox couple near −0.1 V (Fig. S10–S13, ESI†). Complex 2 showed an additional quasi-reversible peak near −1.1 V due to reduction of the BODIPY moiety which was found to be shifted to −0.85 V in complex 3 as a result of iodination in the BODIPY core. Complexes 1–3 are one-electron paramagnetic giving a μeff value of ∼1.8μB for the d9 copper(II) ion.
N) of the pyridine rings of the dipicolylamine ligands (Fig. S7–S9, ESI†). The complexes are redox active showing cyclic voltammograms in DMF-0.1 M [nBu4N](ClO4) for the Cu(II)/Cu(I) redox couple near −0.1 V (Fig. S10–S13, ESI†). Complex 2 showed an additional quasi-reversible peak near −1.1 V due to reduction of the BODIPY moiety which was found to be shifted to −0.85 V in complex 3 as a result of iodination in the BODIPY core. Complexes 1–3 are one-electron paramagnetic giving a μeff value of ∼1.8μB for the d9 copper(II) ion.
| Complex | λmaxa/nm (ε/dm3 mol−1 cm−1) | λema/nm (λexc/nm) [ϕF]b | Efc/V (ΔEp/mV) | ΛMd/S m2 mol−1 | μeffe/μB | Kff/M−1 | Kbg/M−1 | ΔTmh/°C | |
|---|---|---|---|---|---|---|---|---|---|
| DMF | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
||||||||
a Spectra recorded in DMF.b Fluorescence quantum yield values measured in DMF using fluorescein in 0.1 M NaOH as standard (ϕ = 0.79).c In DMF-0.1 M TBAP, Ef = 0.5(Epa + Epc), ΔEp = (Epa − Epc), where Epa and Epc are the anodic and the cathodic peak potentials respectively. The potentials are vs. SCE. Ferrocene as standard. Scan rate = 100 mV s−1.d Molar conductivity data.e Magnetic moment at 298 K using solid powdered samples of the complexes.f Formation constant measured in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O 1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH.g Intrinsic equilibrium binding constant with ct-DNA.h Change in ct-DNA melting temperature in presence of the complexes.i All values in {} are the data for the corresponding ligand system (L1/L2/L3) only. MeOH.g Intrinsic equilibrium binding constant with ct-DNA.h Change in ct-DNA melting temperature in presence of the complexes.i All values in {} are the data for the corresponding ligand system (L1/L2/L3) only. |
|||||||||
| 1 | 246(42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), {267(51 000), {267(51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350)}i, 747(280) 350)}i, 747(280) |
— | −0.16(140) | 24 | 72 | 1.80 | 1.3 × 105 | 3.5 × 105 | 2.0 |
| 2 | 501(70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), {500(73 300), {500(73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650)}i, 677(230) 650)}i, 677(230) |
515(465), [0.14], {510(465), [0.55]}i | −0.1(185), −1.1(90), {−1.12(133)}i | 21 | 77 | 1.82 | 9.6 × 106 | 8.0 × 105 | 4.3 |
| 3 | 535(56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), {533(59 300), {533(59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200)}i, 676(230) 200)}i, 676(230) |
560(515), [0.01], {564(510), [0.01]}i | −0.09(165), −0.84(116), {−0.84(103)}i | 18 | 46 | 1.83 | 4.1 × 106 | 7.6 × 105 | 4.2 |
 | ||
| Fig. 2 Absorption spectra of the complexes 1–3 (a) and emission spectra of 2 and 3 (b) recorded in DMF. Colour codes: complex 1 – black, complex 2 – green, complex 3 – red. | ||
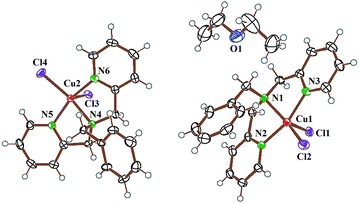
Complex 1 was characterized by single crystal X-ray crystallography. A perspective view of the complex along with the atom labelling scheme is shown in Fig. 3.37 Selected crystallographic parameters, bond distances and angles are given in Tables S1 and S2 (vide ESI†). Complex 1·0.5 Et2O crystallizes in P21/n space group of monoclinic crystal system with diethyl ether as solvent of crystallization (Fig. S14, ESI†). The complex has a mononuclear structure with the metal in a distorted square pyramidal geometry where three nitrogen atoms from benzyldipicolylamine ligand and one chloride ligand are in the equatorial plane with the second chloride ligand occupying the axial position giving a CuN3Cl2 core. There is minor distortion from the square-pyramidal geometry as evidenced from the τ value of 0.11 [τ = 0 for ideal square pyramid (C4v) and τ = 1 for trigonal bipyramid (D3h) structure].38
Conductivity and stability
All the complexes were found to be soluble in common polar organic solvents. They were insoluble in hydrocarbons. Interestingly, complexes 1 and 2 showed significant solubility in water. Complex 3 having the diiodinated BODIPY moiety showed only moderate aqueous solubility possibly due to its molecular weight with di-iodination compared to complex 2, which are otherwise structurally similar. The aqueous solubility of complexes 2 and 3 are significant considering poor aqueous solubility of L2 and L3. The results are of importance making the BODIPY appended Cu(II) complexes better suitable for PDT activity than the dyes alone.The molar conductance study for 1–3 in different solvents gave varied conductance values (Tables 1 and S3, ESI†). The complexes are non-conducting in DMF, DMSO and acetonitrile. In MeOH, the complexes behaved as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte with a loss of one Cl− ligand, as evidenced from the mass spectral data. In dry DMF, complexes were found to be non-conducting. On gradual addition of water to DMF, both 1
1 electrolyte with a loss of one Cl− ligand, as evidenced from the mass spectral data. In dry DMF, complexes were found to be non-conducting. On gradual addition of water to DMF, both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytic behaviours were observed. It is likely that the loss of chloride ligand(s) results to [M(L)Cl(H2O)]+ and [M(L) (H2O)2]2+ species, where L is the dipicolylamine ligand. Complexes 1 and 2 behave as 1
2 electrolytic behaviours were observed. It is likely that the loss of chloride ligand(s) results to [M(L)Cl(H2O)]+ and [M(L) (H2O)2]2+ species, where L is the dipicolylamine ligand. Complexes 1 and 2 behave as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolytes in 50% aq. DMF, while complex 3 shows similar 1
1 electrolytes in 50% aq. DMF, while complex 3 shows similar 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolytic behaviour only at higher water content. Only complexes 1 and 2 displayed 1
1 electrolytic behaviour only at higher water content. Only complexes 1 and 2 displayed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytic properties in 90% aq. DMF. Time dependent UV-visible absorption spectral studies for 1–3 revealed the solution stability of the complexes (Fig. S15–S17, ESI†). The spectra recorded in PBS buffer (pH = 7.2) containing 10% DMSO up to 48 h did not show any significant spectral change suggesting their stability. The formation constants for complexes 1–3 were determined by UV-visible absorption spectral titration using the method of constant total concentration (Table 1, Fig. S18–S20, ESI†). Complex 1 has the stability constant (Kf) value of 1.3 × 105 mol−1. The values for complexes 2 and 3 were 9.6 × 106 and 4.1 × 106 mol−1, respectively. The high Kf values significantly reduces the possibility of any leaching of Cu(II) in the biological media.
2 electrolytic properties in 90% aq. DMF. Time dependent UV-visible absorption spectral studies for 1–3 revealed the solution stability of the complexes (Fig. S15–S17, ESI†). The spectra recorded in PBS buffer (pH = 7.2) containing 10% DMSO up to 48 h did not show any significant spectral change suggesting their stability. The formation constants for complexes 1–3 were determined by UV-visible absorption spectral titration using the method of constant total concentration (Table 1, Fig. S18–S20, ESI†). Complex 1 has the stability constant (Kf) value of 1.3 × 105 mol−1. The values for complexes 2 and 3 were 9.6 × 106 and 4.1 × 106 mol−1, respectively. The high Kf values significantly reduces the possibility of any leaching of Cu(II) in the biological media.
Theoretical studies
DFT computational studies were performed using B3LYP hybrid functional and LANL2DZ basis-set for all the atoms, as incorporated in Gaussian 09 software package for a better understanding of the molecular structure and electronic nature of the complexes.39–41 To ascertain the stationary points, frequency tests were performed after optimisation of the ground-state structures. Energy optimised structures for complexes 1–3 showed distorted square pyramidal geometry of Cu(II) where all the three nitrogen atoms from respective dipicolylamine ligand lie on the basal plane with one chloride ligand, while the second chloride ligand occupies the axial position giving a CuIIN3Cl2 core (Fig. 4, Tables S5–S7, Fig. S21 and S22, ESI†). The energy minimized structures are in accordance with that of the crystal structure of complex 1. For complex 1 the HOMO resides on the orbital of the metal coordinated chloride ligands and the LUMO is on the benzyldipicolylamine moiety. The HOMO and LUMO for 2 and 3 were on the central BODIPY core suggesting the important role of the BODIPY moiety on the photophysical properties of the complexes.Cellular studies
In vitro cytotoxicity of the complexes was measured from the IC50 values using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in human cervical carcinoma HeLa cells and human breast adenocarcinoma MCF-7 cells in dark and on visible light (400–700 nm) exposure (Fig. 5, S23–24, ESI†). The IC50 values of selected metal–BODIPY complexes and non-BODIPY Cu(II) complexes are listed in Tables 2 and S4 (vide ESI†).21,24,42 Complex 1 gave IC50 values of 16.7 and 9.1 μM in HeLa cells in dark and visible light, respectively. Complex 2 having a fluorophoric BODIPY moiety (in L2) gave IC50 values of 12.4 μM in dark and 5.4 μM in light in HeLa cells and 15.6 μM in dark and 6.3 μM in visible light in MCF-7 cells. Complex 3 with an iodinated BODIPY moiety which acts as a photosensitizer showed a remarkable light to dark ratio giving IC50 value of 0.15 μM in light and 11 μM in dark in HeLa cells and 0.17 μM in light and 12.1 μM in dark in MCF-7 cells. The photocytotoxicity of complex 3 in HeLa and MCF-7 cells is remarkable with its nanomolar activity that is about 30 times higher than the FDA approved drug Photofrin®.43 The complexes with IC50 values of ∼10 μM in the dark, however, showed some dark toxicity possibly due to reduction of copper(II) by cellular thiols thus producing ROS. In addition, the dichlorocopper(II) complexes on loss of two chloride ligands can act as DNA crosslinking agents by covalently binding to DNA. | ||
| Fig. 5 MTT assay in HeLa (a) and MCF-7 cells (b) treated with complex 3 for 4 h and 1 h incubation in the dark (black symbol) or on exposure to visible light (400–700 nm, red symbol). | ||
| Entry | HeLa | MCF-7 | ||
|---|---|---|---|---|
| Lightb | Dark | Lightb | Dark | |
| a The structures of the BODIPY metal complexes and non-BODIPY-Cu(II) complexes are shown in Table S4 (vide ESI). The non-PDT Cu(II) complexes reported by Palaniandavar et al. (ref. 42b) and Reedijk et al. (ref. 42c) have IC50 values 3.2 ± 0.3 μM in SiHa cells and 8.6 μM in L1210 cells in dark, respectively.b Visible light of 400–700 nm (dose = 10 J cm−2).c Data from ref. 42a.d Data from ref. 21 (635 nm LED light) in LLC cells.e Data from ref. 24 (400–700 nm visible light).f Data from ref. 43 (633 nm red light). | ||||
| 1 | 9.1 ± 0.3 | 16.7 ± 0.2 | 10.9 ± 0.3 | 16.7 ± 0.3 |
| 2 | 5.4 ± 0.2 | 12.4 ± 0.3 | 6.3 ± 0.1 | 15.6 ± 0.3 |
| 3 | 0.15 ± 0.02 | 11.0 ± 0.2 | 0.17 ± 0.02 | 12.1 ± 0.2 |
| [Pt-BODIPY]c | — | 27.4 ± 1.1 | — | 12.1 ± 2.4 |
| [Ir(II)-BODIPY]d | 2.58 | 9.81 | — | — |
| [Cu(cur) (BODIPY)]e | 3.8 ± 0.2 | 32.1 ± 0.4 | — | — |
| Photofrin®f | 4.3 ± 0.2 | >41 | — | — |
The emissive property of complex 2 was used to study its cellular uptake and localization in the HeLa and MCF-7 cells by confocal microscopy. Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 was used as a nuclear staining dye to ascertain any nuclear uptake of the complexes. The complex (2 μM) showed significant accumulation in the HeLa and MCF-7 cells within 2 h and was retained up to 4 h. The uptake in 4 h was found to be more than that in 2 h. From the merged image, consisting of the green fluorescence of the complex [panels (c and h)] and blue fluorescence of Hoechst 33342 [panels (a and f)], complex 2 was seen to localize mainly in the cytoplasm [panels (d and i)] (Fig. 6 and S25, ESI†). The nuclear morphology of the cells remained intact indicating less toxic nature of the complexes in the dark. The results are indeed of significance since the complexes could serve the dual purpose of imaging the tumor for its detection while remain passive within the cells in absence of light and become cytotoxic on photo-irradiation. Cytoplasmic localization of the complex was further studied using mitochondria tracker red (MTR) in HeLa and MCF-7 cells [panels (b and g)]. The complex did not seem to target mitochondria as evident from lack of any co-localization of the complex and MTR (panels (e and j)) (Fig. 6 and S26, ESI†).
342 was used as a nuclear staining dye to ascertain any nuclear uptake of the complexes. The complex (2 μM) showed significant accumulation in the HeLa and MCF-7 cells within 2 h and was retained up to 4 h. The uptake in 4 h was found to be more than that in 2 h. From the merged image, consisting of the green fluorescence of the complex [panels (c and h)] and blue fluorescence of Hoechst 33342 [panels (a and f)], complex 2 was seen to localize mainly in the cytoplasm [panels (d and i)] (Fig. 6 and S25, ESI†). The nuclear morphology of the cells remained intact indicating less toxic nature of the complexes in the dark. The results are indeed of significance since the complexes could serve the dual purpose of imaging the tumor for its detection while remain passive within the cells in absence of light and become cytotoxic on photo-irradiation. Cytoplasmic localization of the complex was further studied using mitochondria tracker red (MTR) in HeLa and MCF-7 cells [panels (b and g)]. The complex did not seem to target mitochondria as evident from lack of any co-localization of the complex and MTR (panels (e and j)) (Fig. 6 and S26, ESI†).
Annexin-V-FITC/PI assay was performed to study the nature of cell death. Annexin V is a class of phospholipid binding protein which is known to bind phosphatidylserine. The onset of apoptosis mediated cell death results in the flipping of the cell membrane, exposing phosphatidylserine which mainly remains in the inner leaflet of lipid bilayer in healthy cells to the outer leaflet due to a change in the phospholipid asymmetry. This morphological change in cell membrane can then be detected using Annexin-V labelled with FITC in flow cytometry. The red emitting propidium iodide (PI) dye was used as a second marker which is cell membrane impermeable and intercalates to nuclear DNA. Cells undergoing early apoptosis were identified as single positive population for FITC, while necrotic cell population was stained only by PI. The double positive population comprised cells in late apoptosis having compromised cell membrane. To study whether complex 3 is capable of inducing any apoptosis upon visible light irradiation, HeLa cells were treated with 3 (0.2 μM, 4 h incubation) followed by photo-irradiation (1 h) with visible light of 400–700 nm and the assay was performed after 16 h of photo-irradiation. The results are shown in Fig. 7(a). Complex 3 induced apoptosis in ∼29% of HeLa cells upon light irradiation while no significant apoptosis was observed in the dark.
2′,7′-Dichlorofluorescein diacetate (DCFDA) assay was performed to detect formation of any intracellular reactive oxygen species (ROS) in the presence of the complexes. DCFDA is a non-fluorescent and cell permeable dye which on cleavage and oxidation by intracellular esterases and ROS generates fluorescent and cell membrane impermeable DCF. HeLa cells were incubated with complex 3 (0.3 μM) for 4 h in the dark followed by 1 h in visible light (400–700 nm) to perform the experiment. The amount of ROS generated by living cells was then quantified using flow cytometric analysis by measuring the green fluorescence of DCF [λem = 525 nm]. The results indicate a significant shift in ROS production inside the live cells in presence of complex 3 on light exposure, while ROS generated was insignificant in the dark (Fig. 7(b)). To explore the effect of appended iodinated BODIPY moiety, DCFDA assay was also performed with complex 1 (10 μM) which showed only insignificant amount of DCF formation very similar to that generated by DMSO itself after light irradiation (Fig. S27, ESI†). Thus, from the Annexin-V-FITC/PI assay and DCFDA assay data, it can be inferred that the light triggered production of ROS could be responsible for the apoptosis mediated cellular damage highlighting the importance of the photosensitizing BODIPY moiety in 3.
DNA binding and cleavage
DNA binding ability of the complexes was studied with calf thymus (ct) DNA at pH of 7.2 (Tris–HCl buffer, 5 mM) using spectral and hydrothermal methods. The change in absorbance in the UV-visible spectra of the complexes 1–3 was monitored at the respective ligand centred bands with increasing concentration of ct-DNA to determine the intrinsic DNA binding constant (Kb) of the complexes. The data are given in Table 1 (Fig. S28–S30, ESI†).44,45 The DNA binding strength of the complexes follows the order: 2 ≥ 3 > 1. The presence of planar BODIPY core in 2 could facilitate its intercalation using π-stacking interaction between the complex and DNA duplex. Complex 3 having the iodinated BODIPY showed similar binding propensity. Complex 1 which is devoid of the BODIPY moiety showed only moderate DNA binding strength due to absence of any planar moiety with extended conjugation and possibly interacting via groove binding mode.To further investigate the DNA binding mode of the complexes, viscometric titrations were carried out and (η/η0)1/3 vs. [complex]/[DNA] ratio was plotted where η and η0 indicate the relative specific viscosity of ct-DNA in presence and absence of the complexes (Fig. 8(a)).46 The results were compared with that of ethidium bromide (EB) as a classical DNA intercalator which increases the relative viscosity of DNA significantly on binding. Groove binding Hoechst dye did not show any change in the specific viscosity of the DNA solution. It can be inferred from the plot that complexes 2 and 3 have mainly intercalative mode of binding while complex 1 is DNA groove binder. Thermal denaturation studies were performed with ct-DNA to find out DNA melting temperature (Tm) in presence and absence of the complexes. At melting temperature, DNA duplex unwinds to form single strand resulting an increase in absorbance at 260 nm as the base pairs get separated from each other. Binding of the metal complex to DNA normally increases the stability of double helix as a result of additional attractive interactions taking place between the complex and DNA. Intercalating molecules result in better stability to duplex DNA than groove binders and provide a larger positive shift in melting temperature (ΔTm). The ΔTm values for 1–3 are 2.0, 4.3 and 4.2, respectively, suggesting groove binding nature of complex 1 while 2 and 3 are partial DNA intercalators (Fig. 8(b)).
Photo-induced DNA cleavage activity of the complexes was studied using supercoiled (SC) pUC19 DNA (30 μM, 0.2 μg) in Tris–HCl/NaCl (50 mM, pH = 7.2) buffer (Fig. 9a, b and S31, ESI†). Gel electrophoresis was done in agarose gel to estimate the extent of nicked circular (NC) DNA formation from the SC DNA in presence of the copper(II) complexes.47 Monochromatic visible light source of 514 nm (40 mW power) was used from a tuneable continuous-wave (CW) Ar–Kr mixed-gas ion laser. The wavelength chosen for DNA photocleavage is based on the presence of ligand centred electronic spectral band of the complexes 2 and 3 near 500 and 535 nm respectively. Each sample, after treating with the DNA solution in Tris–HCl buffer medium, was kept for 1 h in the dark, followed by 1 h exposure to 514 nm laser light. Complex 1 (20 μM) did not show any significant DNA cleavage in dark and light forming respective 11% and 27% NC DNA from SC DNA, while complex 2 (20 μM) and 3 (5 μM) showed excellent DNA photocleavage activity giving 84% and 91% cleavage of SC DNA to its NC form (Fig. 9(a)). Complexes 2 and 3 did not show any significant DNA cleavage activity in the dark. BODIPY ligands L2 and L3 alone under similar conditions showed 40% and 49% formation of NC DNA on photoactivation, while the values were 10% and 11% respectively in the dark. The results highlight the essential role of Cu(II) in the formation of DNA adducts and the cleavage. The chemical nuclease activity of complexes 1–3 (10 μM) was studied (Fig. 9(a)) in presence of glutathione (GSH, 500 μM). The choice of GSH as reducing agent was based on the presence of a Cu(II)/Cu(I) redox couple in complexes 1–3. All the complexes showed formation of NC DNA ranging from ∼26% to ∼31%. The mechanistic aspects of the DNA photocleavage reactions were probed in the presence of different singlet oxygen quenchers, hydroxyl radical scavengers and SOD as superoxide radical scavenger (Fig. 9(b) and S31, ESI†). Singlet oxygen quenchers NaN3 and TEMP showed inhibition in the DNA photocleavage activity, while there was no significant inhibition in presence of hydroxyl radical (DMSO, KI, catalase) and superoxide radical (SOD) scavengers. The data indicate primarily formation of singlet oxygen as the ROS via type-II process.
Singlet oxygen as ROS
The possibility of production of singlet oxygen was further explored using diphenylisobenzofuran (DBPF) as a standard.48 UV-visible spectral titration was carried out and the change in absorbance of DPBF at ∼414 nm was monitored as a function of time after photoexposure to broadband 400–700 nm light (10 J cm−2 dose) for 5 s each time in presence of a constant concentration of complex 3 (0.1 μM) (Fig. 10). Significant and gradual decrease in the absorption intensity of DPBF indicated photo-induced production of singlet oxygen from complex 3. Singlet oxygen quantum yield measurements were made by an indirect method using Rose Bengal as standard in DMSO (ϕΔ = 0.76).49 A solution of DPBF was photo-irradiated with 3 and the dye separately using a long pass of 530 nm over a time period of 1–10 s.50 The singlet oxygen quantum yield value for 3 (ϕΔ) was found to be 0.53 while it is 0.88 for the iodinated BODIPY ligand (L3) as control (Fig. S32, ESI†). The results support the involvement of cytotoxic singlet oxygen in cellular death by the copper(II) complexes and highlights the importance of the diiodinated BODIPY moiety as a photosensitizer.Experimental section
Materials
Cells used for the biological studies were obtained from the National Centre for Cell Science (NCCS) in Pune, India. Supercoiled (SC) pUC19 DNA (cesium chloride purified) was purchased from Bangalore Genie (India). Calf thymus (ct) DNA, catalase, superoxide dismutase (SOD), 2,2,6,6-tetramethyl-4-piperidone (TEMP), 2,7-dichlorofluorescein diacetate (DCFDA), Hoechst dyes, ethidium bromide (EB), propidium iodide (PI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dulbecco's modified eagle medium (DMEM), Dulbecco's phosphate buffered saline (DPBS) and fetal bovine serum (FBS) were obtained from Sigma (USA). MitoTracker® Deep Red FM (MTR, Cat. no. M22426) was purchased from Invitrogen Bio Services, India.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 787 (280). IR data (cm−1): 3360 (w, br), 3027 (w, br), 2935 (w, br), 2217 (w), 2064 (w), 1950 (w), 1613 (s), 1478 (m), 1438 (s), 1285 (m), 1155 (m), 1025 (m), 865 (m), 753 (s), 702 (m), 650 (m), 509 (m), 480 (m), 425 (m) [w, weak; m, medium; s, strong; br, broad]. Molar conductivity in DMF [ΛM/S cm2 mol−1]: 24. μeff at 298 K: 1.80μB.
000), 787 (280). IR data (cm−1): 3360 (w, br), 3027 (w, br), 2935 (w, br), 2217 (w), 2064 (w), 1950 (w), 1613 (s), 1478 (m), 1438 (s), 1285 (m), 1155 (m), 1025 (m), 865 (m), 753 (s), 702 (m), 650 (m), 509 (m), 480 (m), 425 (m) [w, weak; m, medium; s, strong; br, broad]. Molar conductivity in DMF [ΛM/S cm2 mol−1]: 24. μeff at 298 K: 1.80μB.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700), 372 (1120), 501 (70
700), 372 (1120), 501 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 677 (230). IR data (cm−1): 3410 (w, br), 3353 (w, br), 2923 (w, br), 2180 (w), 2006 (w), 1973 (w), 1613 (w), 1540 (m), 1506 (s), 1469 (s), 1440 (s), 1405 (s), 1368 (m), 1307 (s), 1262 (m), 1193 (s), 1153 (s), 1070 (m), 1049 (s), 1028 (m), 975 (s), 839 (m), 812 (s), 753 (s), 707 (s), 648 (m), 584 (m), 475 (s), 420 (s). Molar conductivity in DMF [ΛM/S cm2 mol−1]: 21. μeff at 298 K: 1.82μB.
300), 677 (230). IR data (cm−1): 3410 (w, br), 3353 (w, br), 2923 (w, br), 2180 (w), 2006 (w), 1973 (w), 1613 (w), 1540 (m), 1506 (s), 1469 (s), 1440 (s), 1405 (s), 1368 (m), 1307 (s), 1262 (m), 1193 (s), 1153 (s), 1070 (m), 1049 (s), 1028 (m), 975 (s), 839 (m), 812 (s), 753 (s), 707 (s), 648 (m), 584 (m), 475 (s), 420 (s). Molar conductivity in DMF [ΛM/S cm2 mol−1]: 21. μeff at 298 K: 1.82μB.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900), 389 (7200), 535 (56
900), 389 (7200), 535 (56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 676 (230). IR data (cm−1): 3421 (w, br), 3387 (w, br), 3046 (w, br), 2913 (w, br), 2205 (w), 2083 (w), 2041 (w), 1960 (w), 1610 (m), 1522 (s), 1441 (s), 1397 (s), 1343 (s), 1302 (s), 1260 (m), 1171 (s), 1117 (s), 1060 (s), 993 (s), 855 (m), 817 (m), 762 (s), 706 (s), 653 (m), 589 (s), 524 (s), 484 (m), 422 (m). Molar conductivity in DMF [ΛM/S cm2 mol−1]: 18. μeff at 298 K: 1.83μB.
300), 676 (230). IR data (cm−1): 3421 (w, br), 3387 (w, br), 3046 (w, br), 2913 (w, br), 2205 (w), 2083 (w), 2041 (w), 1960 (w), 1610 (m), 1522 (s), 1441 (s), 1397 (s), 1343 (s), 1302 (s), 1260 (m), 1171 (s), 1117 (s), 1060 (s), 993 (s), 855 (m), 817 (m), 762 (s), 706 (s), 653 (m), 589 (s), 524 (s), 484 (m), 422 (m). Molar conductivity in DMF [ΛM/S cm2 mol−1]: 18. μeff at 298 K: 1.83μB.Cellular measurements
Conclusions
New BODIPY appended dipicolylamine dichloro copper(II) complexes are designed and prepared as dual action PDT agents. Complex 2 with a green emissive BODIPY unit served as a cellular imaging agent and showed selective cytoplasmic localisation of the complex in the cancer cells. This is important as the nuclear localising chemotherapeutic agents are known to suffer for reduced activity due to NER mechanism to be operative at the nucleus. The present BODIPY complexes thus mimic the cellular localisation properties of the PDT drug Photofrin®. Complex 3 having a diiodinated BODIPY moiety as a photosensitizer exhibited remarkable photocytotoxicity in HeLa and MCF-7 cells. It was found to be ∼30 times more photocytotoxic than Photofrin®. The mechanistic studies revealed apoptotic cell death by 3 on light activated generation of 1O2 as the ROS. The results are of significance in the chemistry of PDT exemplifying metal complexes having BODIPY moieties suitable for cellular imaging and apoptotic cell death on suitable modification of the ligand structure.Conflict of interest
The authors declare no competing interests.Acknowledgements
We thank the Department of Science and Technology (DST, Government of India) for financial support (SR/S5/MBD-02/2007; EMR/2015/000742). A. R. C. thanks the DST for J. C. Bose national fellowship. We are thankful to the Alexander von Humboldt Foundation for donation of an electrochemical system and DST for a CCD diffractometer facility. We thank Dr Sanjoy Mukherjee for his help in the computational studies. We are thankful to Dr D. Ramaiah, Dr J. Joseph and Mr M. Sanmugasundaram of NIIST, Trivandrum, for help in 1O2 quantum yield measurements. A. B. thanks Council for Scientific and Industrial Research (CSIR), New Delhi, for research fellowship. A. D. thanks the University Grants Commission (UGC), New Delhi, for a research fellowship. S. B. thanks IISc, Bangalore, for postdoctoral fellowship. The FACS facility supported by Department of Biotechnology (DBT), Government of India, is acknowledged.Notes and references
- R. Bonnett, Chemical Aspects of Photodynamic therapy, Gordon & Breach, London, UK, 2000 Search PubMed.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- M. Ethirajan, Y. Chen, P. Joshi and R. K Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Ca-Cancer J. Clin., 2011, 61, 250–281 CrossRef PubMed.
- S. H. L. Higgins and K. J. Brewer, Angew. Chem., Int. Ed., 2012, 51, 11420–11422 CrossRef CAS PubMed.
- A. B. Ormond and H. S. Freeman, Materials, 2013, 6, 817–840 CrossRef CAS.
- J. Saczko, M. Mazurkiewicz, A. Chwilkowska, J. Kulbacka, G. Kramer, M. Ługowski, M. Sńietura and T. Banas, Folia Biol., 2007, 53, 7–12 CAS.
- X. Wang and Z. Guo, Chem. Soc. Rev., 2013, 42, 202–224 RSC.
- E. Gabano, M. Ravera and D. Osella, Dalton Trans., 2014, 43, 9813–9820 RSC.
- J. Wezgowiec, M. B Derylo, J. Teissic, J. Orio, M. P. Rols, J. Kulbacka, J. Saczko and M. Kotulska, J. Membr. Biol., 2013, 246, 725–735 CrossRef CAS PubMed.
- K. Berg, J. Golab, M. Korbelik and D. Russell, Photochem. Photobiol. Sci., 2011, 10, 647–648 CAS.
- U. Schatzschneider, Eur. J. Inorg. Chem., 2010, 1451–1467 CrossRef CAS.
- (a) T. Sainuddin, J. McCain, M. Pinto, H. Yin, J. Gibson, M. Hetu and S. A. McFarland, Inorg. Chem., 2016, 55, 83–95 CrossRef CAS PubMed; (b) R. Lincoln, L. Kohler, S. Monro, H. Yin, M. Stephenson, R. Zong, A. Chouai, C. Dorsey, R. Hennigar, R. P. Thummel and S. A. McFarland, J. Am. Chem. Soc., 2013, 135, 17161–17175 CrossRef CAS PubMed.
- R. N. Garner, J. C. Gallucci, K. R. Dunbar and C. Turro, Inorg. Chem., 2011, 50, 9213–9215 CrossRef CAS PubMed.
- N. J. Patel, Y. Chen, P. Joshi, P. Pera, H. Baumann, J. R. Missert, K. Ohkubo, S. Fukuzumi, R. R. Nani, M. J. Schnermann, P. Chen, J. Zhu, K. M. Kadish and R. K. Pandey, Bioconjugate Chem., 2016, 27, 667–680 CrossRef CAS PubMed.
- D. Maggioni, M. Galli, L. D'Alfonso, D. Inverso, M. Vittoria Dozzi, L. Sironi, M. Iannacone, M. Collini, P. Ferruti, E. Ranucci and G. D'Alfonso, Inorg. Chem., 2015, 54, 544–553 CrossRef CAS PubMed.
- (a) J. S. Butler, J. A. Woods, N. J. Farrer, M. E. Newton and P. J. Sadler, J. Am. Chem. Soc., 2012, 134, 16508–16511 CrossRef CAS PubMed; (b) Y. Zhao, J. A. Woods, N. J. Farrer, K. S. Robinson, J. Pracharova, J. Kasperkova, O. Novakova, H. Li, L. Salassa, A. M. Pizarro, G. J. Clarkson, L. Song, V. Brabec and P. J. Sadler, Chem.–Eur. J., 2013, 19, 9578–9591 CrossRef CAS PubMed.
- M. A. Miller, Y. R. Zheng, S. Gadde, C. Pfirschke, H. Zope, C. Engblom, R. H. Kohler, Y. Iwamoto, K. S. Yang, B. Askevold, N. Kolishetti, M. Pittet, S. J. Lippard, O. C. Farokhzad and R. Weissleder, Nat. Commun., 2015, 6, 8692, DOI:10.1038/ncomms9692.
- J. T. F. Lau, P. Lo, W. Fong and D. K. P. Ng, J. Med. Chem., 2012, 55, 5446–5545 CrossRef CAS PubMed.
- S. Banerjee and A. R. Chakravarty, Acc. Chem. Res., 2015, 48, 2075–2083 CrossRef CAS PubMed.
- P. Majumdar, X. Yuan, S. Li, B. L. Guennic, J. Ma, C. Zhang, D. Jacquemin and J. Zhao, J. Mater. Chem. B, 2014, 2, 2838–2854 RSC.
- T. Wang, Y. Hou, Y. Chen, K. Li, X. Cheng, Q. Zhou and X. Wang, Dalton Trans., 2015, 44, 12726–12734 RSC.
- W. Wang, L. Wang, Z. Lia and Z. Xie, Chem. Commun., 2016, 52, 5402–5405 RSC.
- A. Bhattacharyya, A. Dixit, K. Mitra, S. Banerjee, A. A. Karande and A. R. Chakravarty, Med. Chem. Commun., 2015, 6, 846–851 RSC.
- A. Kumar, A. Dixit, S. Banerjee, A. Bhattacharyya, A. Garai, A. A. Karande and A. R. Chakravarty, Med. Chem. Commun., 2016, 7, 1398–1404 RSC.
- T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS PubMed.
- E. Palao, T. Slanina, L. Muchova, T. Solonek, L. Vitck and P. Klan, J. Am. Chem. Soc., 2016, 138, 126–133 CrossRef CAS PubMed.
- J. Tian, J. Zhou, Z. Shen, L. Ding, J. S. Yu and H. Ju, Chem. Sci., 2015, 6, 5959–5977 RSC.
- (a) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC; (b) A. Kamkaew and K. Burgess, J. Med. Chem., 2013, 56, 7608–7614 CrossRef CAS PubMed.
- (a) S. Kolemen, M. Isık, G. M. Kim, D. Kim, H. Geng, M. Buyuktemiz, T. Karatas, X. F. Zhang, Y. Dede, J. Yoon and E. U. Akkaya, Angew. Chem., Int. Ed., 2015, 54, 5340–5344 CrossRef CAS PubMed; (b) I. S Turan, D. Yildiz, A. Turksoy, G. Gunaydin and E. U. Akkaya, Angew. Chem., Int. Ed., 2016, 55, 2875–2878 CrossRef PubMed.
- M. R. Ke, S. L. Yeung, D. K. P. Ng, W. P. Fong and P. C. Lo, J. Med. Chem., 2013, 56, 8475–8483 CrossRef CAS PubMed.
- A. M. Durantini, L. E. Greene, R. Lincoln, S. R. Martínez and G. Cosa, J. Am. Chem. Soc., 2016, 138, 1215–1225 CrossRef CAS PubMed.
- L. Liu, L. Fu, T. Jing, Z. Ruan and L. Yan, ACS Appl. Mater. Interfaces, 2016, 8, 8980–8990 CAS.
- J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, Chem. Soc. Rev., 2015, 44, 8904–8939 RSC.
- C. K. Johnson, ORTEP-III, Report ORNL-5138, Oak Ridge National Laboratory, Oak Ridge, TN, 1976 Search PubMed.
- A. W. Addison and T. N. Rao, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC.
- (a) A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1998, 38, 3098–3100 CrossRef; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 284–298 CrossRef.
- (a) T. Sun, X. Guan, M. Zheng, X. Jing and Z. Xie, ACS Med. Chem. Lett., 2015, 6, 430–433 CrossRef CAS PubMed; (b) R. Loganathan, S. Ramakrishnan, E. Suresh, A. Riyasdeen, M. A. Akbarsha and M. Palaniandavar, Inorg. Chem., 2012, 51, 5512–5532 CrossRef CAS PubMed; (c) P. U. Maheswari, M. V. Ster, S. Smulders, S. Barends, G. P. Wezel, C. Massera, S. Roy, H. D. Dulk, P. Gamez and J. Reedijk, Inorg. Chem., 2008, 47, 3719–3727 CrossRef CAS PubMed.
- E. Delaey, F. Larr, D. D. Vos, A. Kamuhabwa, P. Jacobs and P. D. Witte, J. Photochem. Photobiol., B, 2000, 55, 27–36 CrossRef CAS.
- J. D. McGhee and P. H. Von Hippel, J. Mol. Biol., 1974, 86, 469–489 CrossRef CAS PubMed.
- M. T Carter, M. Rodriguez and A. J. Bard, J. Am. Chem. Soc., 1989, 111, 8901–8911 CrossRef.
- G. Cohen and H. Eisenberg, Biopolymers, 1969, 8, 45–55 CrossRef CAS.
- (a) F. M Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith and K. Struhl, Cur. Protocols in Mol. Biol., John Wiley & Sons, New York, 2003 Search PubMed; (b) J. Bernadou, G. Pratviel, F. Bennis, M. Girardet and B. Meunier, Biochemistry, 1989, 28, 7268–7275 CrossRef CAS PubMed.
- (a) L. M. Rossi, P. R. Silva, L. L. R. Vono, A. U. Fernandes, D. B. Tada and M. S. Baptista, Langmuir, 2008, 24, 12534–12538 CrossRef CAS PubMed; (b) M. Morone, L. Beverina, A. Abbotto, F. Silvestri, E. Collini, C. Ferrante, R. Bozio and G. A. Pagani, Org. Lett., 2006, 8, 2719–2722 CrossRef CAS PubMed; (c) M. Mirenda, C. A. Strassert, L. E. Dicello and E. S. Roman, ACS Appl. Mater. Interfaces, 2010, 2, 1556–1560 CrossRef CAS PubMed.
- N. Gandra, A. T. Frank, O. L Gendre, N. Sawwan, D. Aebisher, J. F. Liebman, K. N. Houk, A. Greerb and R. Gaoa, Tetrahedron, 2006, 62, 10771–10776 CrossRef CAS.
- N. Adarsh, M. Shanmugasundaram, R. R. Avirah and D. Ramaiah, Chem.–Eur. J., 2012, 18, 12655–12662 CrossRef CAS PubMed.
- P. C. Kunz, N. E. Brückmann and B. Spingler, Eur. J. Inorg. Chem., 2007, 394–399 CrossRef CAS.
- Z. Li, Q. Y. Chen, P. D. Wang and Y. Wu, RSC Adv., 2013, 3, 5524–5528 RSC.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
- H. J. Motulsky, Prism 5 Statistics Guide, GraphPad Software Inc., San Diego, CA, 2007, http://www.graphpad.com Search PubMed.
- I. Vermes, C. Haanen, H. Steffens-Nakken and C. Reutelingsperger, J. Immunol. Methods, 1995, 184, 39–51 CrossRef CAS PubMed.
- A. S. Keston and R. Brandt, Anal. Biochem., 1965, 11, 1–5 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization, crystallography and cellular data (Fig. S1–S24, Tables S1–S4). CCDC 1445567. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra23118g |
| This journal is © The Royal Society of Chemistry 2016 |