One pot synthesis of CeO2 nanoparticles on a carbon surface for the practical determination of paracetamol content in real samples†
Mani Sivakumar,
Mani Sakthivel and
Shen-Ming Chen *
*
Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, Taipei 10608, Taiwan. E-mail: smchen78@ms15.hinet.net; Fax: +886-2-2702523; Tel: +886-2-27017147
First published on 17th October 2016
Abstract
Using the effect of interactions between metal oxides and carbon materials is considered an attractive way to make novel composites for electrochemical sensor applications. Both metal oxides and carbon materials have good electrocatalytic properties that can be used for the sensing of different components such as organic/inorganic pollutants, drugs and molecules. These sugar base-derived carbon decorated with CeO2 nanoparticles (CeO2–C) was prepared using a one pot synthesis and used for the electrochemical sensing of paracetamol (PA). The as-prepared CeO2–C was characterized using different techniques and electrochemical studies. The resulting calculated values of sensitivity, linear range and limit of detection were 262 μA mM−1 cm−2, 1 μM to 1.2 mM and 0.07 μM, respectively. Eventually, CeO2–C is predicted to be a suitably active material for practical applications.
1. Introduction
In recent years, a variety of innovative materials have been reported for electrocatalytic applications. Specifically, metal oxides and carbon materials have been considered due to their astonishing physicochemical properties. In the long list of metal oxides, CeO2 was scrutinized by many research groups due to its impressive properties such as efficient photocatalytic activity, large surface area, oxygen ion conductivity, high chemical stability, high specific capacitance, and non-toxicity. Therefore, CeO2 has been used over the past decades in such fields as supercapacitors,1 solar cells,2 fuel cells,3 batteries,4,5 photocatalysis,6 CO oxidation,7 and sensors.8–10 So far a variety of morphologies of CeO2 including nanospheres,11 nanocubes,12 nanoparticles,13 nanorods,14 nanotubes,15 nanowires,16 and nanofibers17 have been reported. Previously, CeO2 has been synthesized using different techniques such as electrospinning, pulsed laser deposition (PVD), electrodeposition, hydrothermal and surfactant assisted synthesis, chemical vapour deposition (CVD) and one pot chemical co-precipitation. Nevertheless, one pot synthesis has been considered as a simple, low temperature and quick process. In general, CeO2 is one of the rare earth metal oxides with a broad band gap around 3.4 eV.18 It has fluorite cubic structure and each Ce4+ ion is surrounded by eight O2− ions in an fcc arrangement, whereas each O2− ion is tetrahedrally surrounded by four Ce4+ ions.19,20 It exhibits an extraordinary capacity to store or release oxygen depending upon its variation in oxidation state between +3 and +4.21 Moreover, its electrocatalytic properties can be tuned depending on the quantity of adsorbent on its surface.8 In addition, negatively charged biomolecules can be effectively immobilized on positively charged CeO2.22 For example, Wei Zhang et al. used CeO2 nanoshuttles for DNA immobilization to probe a cancer gene,23 Debao Wang et al. synthesized CeO2 decorated Ni(OH)2 for a highly sensitive H2O2 sensor,24 Jiewu Cui et al. produced NiO/CeO2 hybrid nanoflakes for a glucose sensor,48 and R. M. Shereema et al. synthesized CeO2/MWCNT for the sensing of acetaldehyde,25 etc. These examples confirm that CeO2 possesses the biocompatibility necessary for biomolecule immobilization. Furthermore, carbon materials enhance the electrochemical behaviour of CeO2 (ref. 9 and 10) due to their extensive electrochemical activity, extraordinary surface area and conductivity.26,27On the other hand, there are several carbon allotropes such as fullerene, CNT, and graphene. Contemporary alternative carbon materials to these carbon allotropes are expected to be developed. For example, prominent sugar bases (glucose, sucrose, fructose etc.) derived from carbon were reported recently.28–30 These sugar base-derived carbons have high catalytic activity, improved surface area, unique specific capacitance, considerable chemical stability and outstanding electrical conductivity.31–37 Therefore this kind of carbon material with these substantial properties can enhance the properties of CeO2 as well as those of carbon allotropes.
The continuous consumption of pharmaceutical drugs can affect the human health. Particularly, PA is widely used as a pharmaceutical for chronic pain, headaches, postoperative pain and backache. However, overdose of PA can result in many side effects such as diarrhea, increased sweating, stomach cramps and a loss of appetite etc.38–40 Therefore, a quick and effective method is need to measure the level of PA usage. Herein, we report an electrochemical sensing method, which is low cost, and has high sensitivity, selectivity and reproducibility.
In this work we have reported CeO2 nanoparticles decorated onto a sucrose derived carbon surface (CeO2–C) and the corresponding modified glassy carbon electrode as an active probing electrode for the electrochemical detection of PA. To the best of our knowledge, this is the first time the integration of CeO2 onto a sucrose derived carbon surface has been achieved by a simple one pot synthesis without high temperatures.
2. Experimental
2.1 Materials and methods
Cerium(III) nitrate hexahydrate (CeNO3·6H2O) was purchased from Alfa Aesar, and sucrose and ethanol were purchased from Sigma Aldrich. A human blood sample was purchased in Chang Gung Memorial Hospital and under the moral ethics committee of Taiwan. All chemicals were used without further purification.2.2 Synthesis of cerium oxide–carbon (CeO2–C)
The one-pot synthesis of CeO2–C was conducted using a simple hydrothermal method. Briefly, the sucrose was dissolved in 10 mL of ethanol and distilled water using ultra-sonication for 10 min. Further, cerium(III) nitrate hexahydrate solution (5 mM, 10 mM and 15 mM) was added to the above sucrose solution and stirred for 1 h. Then, the resultant gel solution was heated at 120 °C for 15 h. After 15 h, the obtained black precipitate was washed several times with distilled water and ethanol. Then it was subjected to a drying process in an air oven. Finally, the black powder was calcined at 350 °C for 3 h as shown in Scheme 1.2.3 Characterization
The surface morphology and structural information were recorded using scanning electron microscopy (JEOL) and X-ray diffraction (XRD) using a Rigaku MiniFlex LL. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were performed using CHI instruments (CHI900, CHI611A). A three electrode system was used with the modified glassy carbon electrode (GCE) as the working electrode, Ag/AgCl as the reference electrode (saturated with KCl), and Pt wire as the counter electrode.2.4 Electrode preparation and electrochemical measurements
To start, the one pot synthesized CeO2–C and CeO2 powders (5 mg) were dispersed in ethanol under ultra-sonication for 1 h. Then, the glassy carbon electrode (GCE) was polished with alumina slurry and rinsed with distilled water and ethanol. 6 μL of the resultant suspension was drop casted onto the polished GCE surface and dried in an air oven. This same process was followed for all further electrochemical experiments. Cyclic voltammograms (CV) and differential pulse voltammetry (DPV) studies were executed after purging with nitrogen gas into a 0.1 M PBS solution (pH 7) at room temperature before starting each measurement.2.5 Real sample preparations
A human blood sample was prepared as previously reported in the literature.57 The human serum sample analysis and experimental protocol have been approved by the Institutional Animal Ethics Committee. Experiments with the real samples were performed in compliance with the laws and institutional guidelines of Chang Gung University, Taiwan.3. Results and discussion
3.1 Morphological and structure characterizations
The as-prepared CeO2 and CeO2–C morphologies were examined using scanning electron microscopy (SEM) and are shown in Fig. 1. The as-synthesized CeO2 displayed a nanorod-like structure, as shown in Fig. 1(A). The corresponding EDX spectrum is shown in Fig. S1C,† which implies the considerable presence of CeO2 in the sample, as is illustrated by the weight percentages of the constituents shown in Fig. S1B.†On other hand, SEM was performed for the CeO2–C composite. Here, it can be clearly seen in Fig. 1(B) and (C) that the CeO2 nanoparticles have decorated the carbon surface. The corresponding SEM affiliated EDX spectrum is shown in Fig. 1(D). Fig. S2(A) and (B)† show the SEM images for low and high concentrations of cerium oxide in the nanocomposites to study the effect of the concentration of the cerium precursor. From this result, a few micro-sized particles and aggregated particles were clearly observed corresponding to the low and high concentrations, respectively. Then, the EDX spectrum of the CeO2–carbon composite was used to confirm the presence of cerium, oxygen and carbon with high purities and with a reasonable weight percentage. Here, sucrose was used as both the carbon source and as a reducing agent. Sucrose naturally exists with carbohydrates. Hence, the sucrose was exposed to 186 °C to transform the carbonaceous nanoparticles into the nanocomposite.41
Fig. 2(A) shows the XRD pattern of the CeO2–C nanocomposite and its corresponding diffraction lattice planes are (111), (200), (220), (311), (200), (400) and (331). This result demonstrates the absence of impurity peaks and was used to predict the purity of the reported nanocomposites.42 In the diffraction pattern, a small peak appears at 23°, which implies the integration of carbon into the nanocomposite material. The well crystalline nature of the nanocomposite was confirmed by its high-intensity peaks in the XRD spectrum. Moreover, the diffraction patterns were analyzed with comparison to the well-matched standard JCPDS card no. 65-5923 for CeO2. The XRD pattern was observed with a low intensity of carbon, which is not significant due to the small quantities of carbon. Finally, the diffraction peaks were used to confirm the composition and crystalline purity of the nanocomposites.
The FT-IR spectra of the CeO2 and CeO2–C nanocomposites are shown in Fig. 2(B). The band at 3600 cm−1 indicates the O–H stretching of surface adsorbed water and hydroxyl groups. Further, the spectrum displayed a Ce![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 450 cm−1 for CeO2. The absorption band at 1026 cm−1 denotes the corresponding stretching vibration for C
O stretching band at 450 cm−1 for CeO2. The absorption band at 1026 cm−1 denotes the corresponding stretching vibration for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The resultant carbon band confirmed the formation of the nanocomposite and the small amount of carbon present.43–46 Raman spectroscopy studies were performed to identify CeO2 and CeO2–C from their observed fingerprint as shown in Fig. 3(A). The spectrum was recorded for these materials within the range of 0 to 2000 cm−1. The disorder and the in-plane vibration of the sp2 carbons of the graphitic carbon were established due to the presence of D and G bands at 1353.32 cm−1 and 1574.22 cm−1, respectively.46 The inset of Fig. 3(A) shows the spectrum for pure cerium oxide with peaks at 476 cm−1 and 594 cm−1 due to the second order transverse acoustic of CeO2. The same peaks are present in the nanocomposite but the intensity is less due to the presence of carbon. Hence, the spectrum confirmed the presence of a small amount of carbon in the composite.
O. The resultant carbon band confirmed the formation of the nanocomposite and the small amount of carbon present.43–46 Raman spectroscopy studies were performed to identify CeO2 and CeO2–C from their observed fingerprint as shown in Fig. 3(A). The spectrum was recorded for these materials within the range of 0 to 2000 cm−1. The disorder and the in-plane vibration of the sp2 carbons of the graphitic carbon were established due to the presence of D and G bands at 1353.32 cm−1 and 1574.22 cm−1, respectively.46 The inset of Fig. 3(A) shows the spectrum for pure cerium oxide with peaks at 476 cm−1 and 594 cm−1 due to the second order transverse acoustic of CeO2. The same peaks are present in the nanocomposite but the intensity is less due to the presence of carbon. Hence, the spectrum confirmed the presence of a small amount of carbon in the composite.
 | ||
| Fig. 3 (A) Raman spectra of CeO2 (inset) and CeO2–C. (B) EIS spectra for the bare GCE, CeO2, and CeO2–C in 5 mM of [Fe (CN)6]3−/4− in 0.1 M KCl solutions. Inset: Randles equivalence circuits. | ||
Fig. 3(B) shows the EIS spectrum of the different modified electrodes in 0.1 M KCl solution containing [Fe(CN)6]3−/4− supporting electrolytes. The measurements were recorded in the frequency range of 100 kHz to 100 Hz. The semicircle at the lower frequency region corresponds to the electron-transfer limited process (Rct), and the higher frequency region reveals the electrolyte diffusion process. In these results, the Rct values of the CeO2–C/GCE, CeO2/GCE and bare GCE electrodes were 39.65 Ω, 164.82 Ω and 582.69 Ω, respectively. A lower Rct was obtained for the CeO2–C composite due to its higher conductivity compared to the CeO2 nanorods and GCE. The vertical area nearly parallel to the imaginary axis accounts for the developed performance of the nanocomposite.
3.2 Electrochemical studies of CeO2–C/GCE
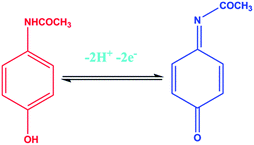
The electrochemical performance of the different modified electrodes was observed in the presence and absence of PA in N2 saturated 0.1 M PBS (pH 7.2) at a scan rate of 50 mV s−1. The CV curves of the electrodes represented by (a) unmodified GCE, (b) CeO2/GCE, (c′) CeO2–C/GCE with PA and a blank measurement for CeO2–C/GCE (c) are shown in Fig. 4(A). It is worth nothing that the PA redox peak current for CeO2–C/GCE was two-fold higher than that for CeO2 and the unmodified GCE electrode. The modified CeO2–C/GCE electrode successfully participated in the reversible electrochemical redox process of PA, as illustrated in the scheme below:The above scheme illustrates the two electron and two proton process involved in the oxidation and reduction of PA. The demands of the PA sensor include a high electrochemically active surface area and excellent electrocatalytic activity for well-defined redox peaks. Finally, the prompt and instantly reversible redox behavior of the CeO2–C nanocomposite towards PA was confirmed from the cyclic voltammetry curve shown in Fig. 4(A), illustrating the unique physicochemical properties and enhanced electrocatalytic activity at the CeO2–C/GCE surface.47 Fig. 4(B) shows the CV curve of the CeO2–C/GCE electrode at different scan rates in the presence of PA (196 μM) in N2 saturated 0.1 M PBS (pH 7.2). The linear regression equation was obtained from the graph of the square root of scan rate vs. peak current to give a calibration coefficient value of about R2 = 0.9964. The above reported results suggested that the diffusion control process participates in the kinetics of the overall redox process of PA. The charge transfer coefficient value was calculated as α = 0.52, which was derived from the Tafel plot for the scan rate of 50 mV s−1 by substituting the value of the slope 7.9496 V−1 decade in the following equation: n(1 − α)F/2.3RT, as is shown in Fig. S3.†49
3.3 The effect of PA concentration and pH of the electrolyte
Fig. 5(A) shows the CV curve of the CeO2–C/GCE electrode towards the detection of PA with an increasing redox peak current with respect to the PA concentration in the range of 49 to 698 μM. The PA oxidation signal increased linearly with respect to the PA concentration and the peak potential was slightly shifted towards the positive side, which might be related to the formation of an adsorption layer of PA on the CeO2–C/GCE surface so that the adsorption layer expands the size of the diffusion layer above the electrode surface. Moreover, the linear calibration coefficient value was calculated to be about R2 = 0.9979 (Fig. 5(A) above inset).The pH of the electrolyte is an important chemical parameter, because it changes the reduction and oxidation potentials of the preferred analytes. An equal number of protons and electrons are involving in the surface reaction of an active material and any analytes. Knowledge about progressive changes in the pH of an electrolyte and the redox potential is important for the growing field of electrochemical sensors. Based on this subject, Fig. 5(B) shows the electrolyte pH effect on the CV curve of the CeO2–C/GCE electrode. The peak potential shifted to the negative side with respect to the change in pH of the electrolyte from 3 to 11. PA exhibited a good redox response in curve (i) and curve (ii) at pH 7.2, as concluded from the plot of pH vs. peak current (inset: curve (i)). Similarly, the peak potential vs. current plot was drawn and the electron transfer coefficient value was calculated to be about R2 = 0.985. The CeO2–C/GCE electrode shows a redox peak potential of about E0 = 0.44 V (inset: curve (ii)) at pH 7.2, which was comparatively more noticeable than at other pHs. The regression equation of pH vs. current was calculated as Ep (V) = −0.044 (pH) + 0.842 (R2 = 0.9862) and the relative slope value was calculated to be about 44 mV per pH unit, which was slightly lower than the theoretical value (59 mV pH−1). Therefore, the modified electrode surface demonstrated that the determined electron-transfer process occurred during the oxidation of PA with an equal number of protons involved in the process.
3.4 DPV studies
The CeO2–C/GCE composite was subjected to the stepwise addition of PA between a defined set of potentials in a DPV study for the determination of the primary electrochemical parameters important for sensors such as LOD, linear range and sensitivity. Fig. 6 shows the oxidation of PA at the CeO2–C/GCE electrode with increasing concentration of PA from 1 μM to 1.2 mM while the peak currents are linearly increased. Consequently, the plot for PA concentration vs. peak currents was drawn and the regression equation was calculated with a coefficient value of about R2 = 0.9963. From this experiment, the CeO2–C/GCE electrode was observed to have good sensitivity and accessible detection limits of about 262 μA mM−1 cm−2 and 0.07 μM, respectively. These electrochemical parameters of this reported modified electrode were compared with other different modified electrodes as shown in Table 1. Hence, the CeO2–C/GCE electrode was considered to be more suitable for practical application. | ||
| Fig. 6 DPV curves for CeO2–C modified GCE with different additions of paracetamol (from 1 μM to 1.2 mM) in 0.1 M PBS (pH 7.2) at a scan rate of 50 mV s−1. | ||
| Modified electrodes | Techniques | Linear ranges (μM) | Sensitivity (μA mM−1) | LOD (μM) | Ref. |
|---|---|---|---|---|---|
| (ERGO)/NHCF | LSV | — | 531 | 14.22 | 50 |
| Carbon-coated nickel nanoparticles/GCE | DPV | 7.8–110 | — | 2.3 | 51 |
| Nafion/TiO2-GR/GCE | DPV | 0.1–100 | — | 0.21 | 52 |
| MWNT/GCE | DPV | 3–300 | — | 0.8 | 53 |
| PEDOT/GO/GCE | DPV | — | — | 0.57 | 54 |
| AuNP–PGA/SWCNT | DPV | 8.3–145.6 | — | 1.18 | 55 |
| PSi/Pd-NS/CNTPE | DPV | 1.0–700 | 44.9 | 0.4 | 56 |
| CeO2–C/GCE | DPV | 1–1219 | 262 | 0.07 | This work |
3.5 Reproducibility, selectivity and stability
Stability, repeatability and reproducibility studies were used to identify the suitability of the modified electrode for sensor application. The reproducibility, selectivity and stability of the PA sensor were examined for the proposed CeO2–C/GCE modified electrode. To estimate the reproducibility three independent CeO2–C/GCE modified electrodes were prepared and the current responses were obtained in the presence of PA (296 μM). From the results, the relative standard deviation (RSD) was calculated to be about 2.4%, which confirmed that the fabricated electrodes are highly reproducible. To investigate the selectivity of the CeO2–C/GCE modified electrode for the determination of PA, CV measurements were performed in the presence of various interfering agents (ascorbic acid (AA), dopamine (DA), uric acid (UA), glucose and sulfite) as shown in Fig. S4.† The 2-fold peak current responses for PA are instantly higher than that of the common interfering species with equal concentrations present in the studies. This result was related to the exclusive selectivity of the CeO2–C modified electrode for PA. Furthermore, a stability test was carried out by storing the CeO2–C/GCE modified electrode at room temperature. After 4 weeks 92% of the initial value of the PA oxidation peak current was retained under the same conditions. Hence, the CeO2–C modified electrode was suitable for practical application.3.6 Real sample analysis
This research aims to prepare an electrochemically active material for practical applications. Hence, the CeO2–C/GCE electrode was used to study real samples using paracetamol tablets and a human serum sample. Typically, 2 mg of paracetamol powder sample was dispersed in 0.1 M PBS (pH 7.2) and the resultant solutions were centrifuged before the test. The human blood sample was prepared as previously reported in the literature.57 The human serum sample was prepared using a standard addition method where the human serum sample (200 μL) with PA (4 mg) was freshly prepared 1 h before the test.The response values for the paracetamol and human serum solution were observed and found by electrochemical methods as shown in Fig. 7(A) and (B). Eventually, the proposed CeO2–C/GCE electrode was defined as being applicable for practical real sample analysis.
 | ||
| Fig. 7 (A and B) CV curves of the real sample analysis in 0.1 M PBS (pH 7.2) at a scan rate of 50 mV s−1. | ||
4. Conclusion
In this work, a CeO2–C nanocomposite material was successfully prepared by a one pot easy synthesis method and developed as an electrochemically active material and as an exclusive PA sensor. The CeO2–C nanocomposite was found to have a high electrocatalytic activity towards the redox reaction of PA with good selectivity, stability and reproducibility, including a lower electron transfer resistance than other known composites. In addition, the developed electrode was examined for practical application to detect a paracetamol tablet and is expected to reach the prototype level of sensor.Acknowledgements
Financial support for this work was given by the Ministry of Science and Technology, Taiwan NSC101-2113-M-027-001-MY3 to SMC and is gratefully acknowledged.References
- H. Zhang, J. Gu, J. Tong, Y. Hu, B. Guan, B. Hu, J. Zhao and C. Wang, Chem. Eng. J., 2016, 286, 139–149 CrossRef CAS.
- J. Roh, S. H. Hwang and J. Jang, ACS Appl. Mater. Interfaces, 2014, 6, 19825–19832 CAS.
- Y. Dong, D. Li, X. Feng, X. Dong and S. Hampshire, RSC Adv., 2013, 3, 17395–17401 RSC.
- T. Montini, M. Melchionna, M. Monai and P. Fornasiero, Chem. Rev., 2016, 116, 5987–6041 CrossRef CAS PubMed.
- H. Liu and H. Liu, J. Alloys Compd., 2016, 681, 342–349 CrossRef CAS.
- A. Umar, R. Kumar, M. S. Akhtar, G. Kumar and S. H. Kim, J. Colloid Interface Sci., 2015, 454, 61–68 CrossRef CAS PubMed.
- J. Zhang, Y. Cao, C. A. Wang and R. Ran, ACS Appl. Mater. Interfaces, 2016, 8, 8670–8677 CAS.
- N. Lavanya, C. Sekar, R. Murugan and G. Ravi, Mater. Sci. Eng., C, 2016, 65, 278–286 CrossRef CAS PubMed.
- Y. Wei, Q. A. Huang, M. G. Li, X. J. Huang, B. Fang and L. Wang, Electrochim. Acta, 2011, 56, 8571–8575 CrossRef CAS.
- S. Yang, G. Li, G. Wang, L. Liu, D. Wang and L. Qu, J. Alloys Compd., 2016, 688, 910–916 CrossRef CAS.
- X. Ouyang, W. Li, S. Xie, T. Zhai, M. Yu, J. Gan and X. Lu, New J. Chem., 2013, 37, 585–588 RSC.
- X. Xiao, Q. Luan, X. Yao and K. Zhou, Biosens. Bioelectron., 2009, 24, 2447–2451 CrossRef CAS PubMed.
- M. K. Mohanapriya, K. Deshmukh, M. B. Ahamed, K. Chidambaram and S. K. K. Pasha, Mater. Today, 2016, 3, 1864–1873 CrossRef.
- Z. K. Ghouri, N. A. M. Barakat, H. Y. Kim, M. Park, K. A. Khalil, M. H. E. Newehy and S. S. A. Deyab, Arabian J. Chem., 2016, 9, 219–228 CrossRef CAS.
- T. Wang, L. Zhang, J. Zhang and G. Hua, Microporous Mesoporous Mater., 2013, 171, 196–200 CrossRef CAS.
- X. H. Lu, D. Z. Zheng, J. Y. Gan, Z. Q. Liu, C. L. Liang, P. Liu and Y. X. Tong, J. Mater. Chem., 2010, 20, 7118–7122 RSC.
- Y. Liu, Y. Ding, L. Zhang, P. X. Gao and Y. Lei, RSC Adv., 2012, 2, 5193–5198 RSC.
- A. K. Bhosale, N. L. Tarwal, P. S. Shinde, P. M. Kadam, R. S. Patil, S. R. Barman and P. S. Patil, Solid State Ionics, 2009, 180, 1324–1331 CrossRef CAS.
- T. Mori, Y. Wang, J. Drennan, G. Auchterlonie, J. G. Li and T. Ikegami, Solid State Ionics, 2004, 175, 641–649 CrossRef CAS.
- M. Dudek, J. Eur. Ceram. Soc., 2008, 28, 965–971 CrossRef CAS.
- C. Lee, Q. L. Meng, H. Kaneko and Y. Tamaura, Int. J. Hydrogen Energy, 2013, 38, 15934–15939 CrossRef CAS.
- F. F. Deng, C. Ding, Y. Wang, W. T. Li, L. L. Liu and H. Li, Anal. Methods, 2014, 6, 9228–9233 RSC.
- W. Zhang, T. Yang, X. Zhuang, Z. Guo and K. Jiao, Biosens. Bioelectron., 2009, 24, 2417–2422 CrossRef CAS PubMed.
- D. Wang, L. Pang, H. Mou, Y. Zhou and C. Song, RSC Adv., 2015, 5, 24101–24109 RSC.
- R. M. Shereema, S. R. Nambiar, S. S. Shankar and T. P. Rao, Anal. Methods, 2015, 7, 4912–4918 RSC.
- F. Tehrani and B. Bavarian, Sci. Rep., 2016, 6, 27975 CrossRef CAS PubMed.
- S. K. Lee, M. J. Song, J. H. Kim, T. S. Kanl, Y. K. Lim, J. P. Ahn and D. S. Lim, NPG Asia Mater., 2014, 6, 115 CrossRef.
- J. C. Heckel, F. F. Farhan and G. Chumanov, Colloid Polym. Sci., 2008, 286, 1545–1552 CAS.
- H. Guo, X. Qi, L. Li and R. L. Smith Jr, Bioresour. Technol., 2012, 116, 355–359 CrossRef CAS PubMed.
- L. Mao, Y. Zhang, Y. Hu, K. H. Ho, Q. Ke, H. Liu, Z. Hu, D. Zhao and J. Wang, RSC Adv., 2015, 5, 9307–9313 RSC.
- L. Wei and G. Yushin, Carbon, 2011, 49, 4830–4838 CrossRef CAS.
- C. Wu, J. Xu, J. Ding, N. Yuan, P. Yan, R. Zhang and H. Liu, Nano, 2016, 7, 1650075 CrossRef.
- J. Y. Liang, C. C. Wang and S. Y. Lu, J. Mater. Chem. A, 2015, 3, 24453–24462 CAS.
- M. Taleb, J. Nerut, T. Tooming, T. Thomberg, A. Jänes and E. Lust, J. Electrochem. Soc., 2015, 162, 651–660 CrossRef.
- M. Amjadi, R. Shokri and T. Hallaj, Luminescence, 2016, 1–6 Search PubMed.
- R. R. Devarapalli, S. Szunerits, Y. Coffinier, M. V. Shelke and R. Boukherroub, ACS Appl. Mater. Interfaces, 2016, 8, 4298–4302 CAS.
- Z. H. Miao, H. Wang, H. Yang, Z. Li, L. Zhen and C. Y. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 15904–15910 CAS.
- Y. Fan, J. H. Liu, H. T. Lu and Q. Zhang, Colloids Surf., B, 2011, 85, 289–292 CrossRef CAS PubMed.
- X. Kang, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. Lin, Talanta, 2010, 81, 754–759 CrossRef CAS PubMed.
- A. Kutluay and M. Aslanoglu, Anal. Chim. Acta, 2014, 839, 59–66 CrossRef CAS PubMed.
- D. Yuan, X. Yuan, W. Zou, F. Zeng, X. Huang and S. Zhou, J. Mater. Chem., 2012, 22, 17820–17826 RSC.
- Y. He, X. Liang and B. Chen, Nano Res., 2015, 8, 1269–1278 CrossRef CAS.
- E. K. Goharshadi, S. Samiee and P. Nancarrow, J. Colloid Interface Sci., 2011, 356, 473–480 CrossRef CAS PubMed.
- M. L. D. Santos, R. C. Lima, C. S. Riccardi, R. L. Tranquilin, P. R. Bueno, J. A. Varela and E. Longo, Mater. Lett., 2008, 62, 4509–4511 CrossRef.
- N. Padmanathan and S. Selladurai, Ionics, 2014, 20, 409–420 CrossRef CAS.
- D. Yang, P. Liu, Y. Gao, H. Wu, Y. Cao, Q. Xiao and H. Li, J. Mater. Chem., 2012, 22, 7224–7231 RSC.
- R. Kumar and P. Bhargava, RSC Adv., 2016, 6, 8705–8713 RSC.
- J. Cui, J. Luo, B. Peng, X. Zhang, Y. Zhang, Y. Wang, Y. Qin, H. Zheng, X. Shua and Y. Wu, Nanoscale, 2016, 8, 770–774 RSC.
- S. Cheraghi, M. A. Taher and H. K. Maleh, Electroanalysis, 2016, 28, 366–371 CrossRef CAS.
- B. Devadas, M. Rajkumar, S. M. Chen and R. Saraswathi, Int. J. Electrochem. Sci., 2012, 7, 3339–3349 Search PubMed.
- S. Wang, F. Xie and R. Fei Hu, Sens. Actuators, B, 2007, 123, 495–500 CrossRef CAS.
- Y. Fan, J. H. Liu, H. T. Lu and Q. Zhang, Colloids Surf., B, 2011, 85, 289–292 CrossRef CAS PubMed.
- Z. A. Alothman, N. Bukhari, S. M. Wabaidur and S. Haider, Sens. Actuators, B, 2010, 146, 314–320 CrossRef CAS.
- W. Si, W. Lei, Z. Han, Y. Zhang and Q. Hao, Sens. Actuators, B, 2014, 193, 823–829 CrossRef CAS.
- M. P. N. Bui, C. Ai Li, K. N. Han, X. H. Pham and G. H. Seong, Sens. Actuators, B, 2012, 174, 318–324 CrossRef CAS.
- A. A. Ensafi, N. Ahmadi, B. Rezaei and M. M. Abarghoui, Talanta, 2015, 134, 745–753 CrossRef CAS PubMed.
- B. Devadas, S. Cheemalapati, S. M. Chen, M. Ajmal Ali and F. M. A. Al-Hemaid, Ionics, 2015, 21, 547–555 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23114d |
| This journal is © The Royal Society of Chemistry 2016 |