Silanization induced inherent strain in graphene based filler influencing mechanical properties of polycarbonate urethane nanocomposite membranes†
Abstract
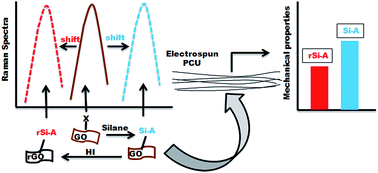
Nanosheet type fillers apart from their size and surface functional groups may have numerous attributes affecting the mechanical properties of polymeric nanocomposites. To study these, silane-functionalized graphene based fillers were synthesized by chemically grafting N-[3-(trimethoxysilyl)propyl]diethylenetriamine (TMPT) onto graphene oxide (GO) and carboxylated GO (GOCO) using different chemistries. Their respective silanization yielded nano-fillers with amine (GOSAM) and alkoxy (GOCSAL) groups. Further, hydroiodic acid (HI) treatment led to synthesis of their reduced counterparts GRSAM and GRCSAL. The resulting TMPT-functionalized nanosheets were characterized by Fourier transform infrared spectroscopy (FT-IR) confirmed silane functionalization. A blue shift in Raman spectra indicated that during silanization with different terminal groups an inherent compressive strain has developed, while reduction with HI caused a red shift indicating a tensile strain, in these nanosheets. Polycarbonate urethane nanocomposite electrospun membranes (PCU) incorporated with these respective fillers at different loadings were analyzed. Morphology of the nanocomposite membranes was observed under SEM and membranes were characterized by static and dynamic mechanical analysis. The study indicated that the exfoliation and dispersion of graphene sheets in PCU has significantly improved due to surface functionalization while it also exhibited a novel aspect, variations in their mechanical properties in respect to the type of strain present in incorporated nanosheet fillers. The nanosheet fillers with compressive strain contributed more to the mechanical property enhancement of nanocomposite membranes, than the fillers with tensile strain. A spring and molecule model was thus proposed as possible explanation to relate inherent strain in filler to that of nanocomposite membrane mechanical properties. In vitro non-cytotoxic and hemocompatible nature of these fibroporous nanocomposite membranes provided their potential in biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...