 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stable nickel-substituted spinel cathode material (LiMn1.9Ni0.1O4) for lithium-ion batteries obtained by using a low temperature aqueous reduction technique
Niki
Kunjuzwa
ab,
Mesfin A.
Kebede
*a,
Kenneth I.
Ozoemena
ab and
Mkhulu K.
Mathe
*a
aEnergy Materials, Materials Science and Manufacturing, Council for Scientific and Industrial Research (CSIR), Pretoria, 0001, South Africa. E-mail: kmathe@csir.co.za; mkebede@csir.co.za; Fax: +27 128412135; Tel: +27 128413665 Tel: +27 128413588
bMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Private Bag 3, P O WITS 2050, Johannesburg, South Africa
First published on 21st November 2016
Abstract
A nickel substituted spinel cathode material (LiMn1.9Ni0.1O4) with enhanced electrochemical performance was successfully synthesized by using a locally-sourced, low-cost manganese precursor, electrolytic manganese dioxide (EMD), and NiSO4·6H2O as a nickel source by means of a low temperature aqueous reduction synthesis technique. This synthesis protocol is convenient to scale up the production of the spinel cathode material, with minimal nickel content (Ni = 0.1) in the structure, for lithium-ion battery applications. Ni-ions substituting Mn-ions was confirmed using XRD, EDS, XPS and electrochemical performance studies. LiMn1.9Ni0.1O4 materials showed an octahedral shape with clearly exposed (111) facets that enhanced the Li-ion kinetics and improved the cycling performance compared to the pristine spinel sample (LiMn2O4). The LiMn1.9Ni0.1O4 sample exhibited superior capacity retention by retaining 84% of its initial capacity (128 mA h g−1) whereas pristine LiMn2O4 retained only 52% of its initial capacity (137 mA h g−1). XPS confirmed that the Mn3+/Mn4+ ratio changed with nickel substitution and favored the suppression of capacity fading. The study clearly suggests that the integration of small amounts of Ni into the spinel structure is able to eliminate the disadvantageous Jahn–Teller effects in the LiMn2O4.
Introduction
Lithium-ion battery (LIB) technology is well-developed for portable electronic devices (like cellphones, laptops, iPads, etc.) which have been widely used. However, to implement LIBs for large-scale high-power systems such as plug-in hybrid electric vehicles (PHEVs) or plug-in electric vehicles (PEVs), there is a great need to increase the energy and power capabilities of these batteries.1–5 Nickel-substituted LiMn2O4 (i.e., LiMn2−xNixO4) has emerged as one of the promising spinel cathode materials for lithium-ion batteries. A member of the family is the high-voltage spinel LiMn1.5Ni0.5O4 (LMNO) is considered as one of the most promising cathode materials for Li-ion batteries.6,7 In comparison with the commercial LiCoO2 positive electrode, LiMn1.5Ni0.5O4 has been shown to intercalate–deintercalate Li+ ions at very high potential (E = 4.7 V vs. Li+/Li).8 It has a large high intrinsic rate capability offered by the 3-dimensional lithium-ion diffusion in the spinel lattice. Besides, it is much safer, low-cost, and greener.9 There is a continued need to reduce the cost of the LiMn1.5Ni0.5O4 by the use of low-cost synthesis method, the use of low-cost manganese precursor (such as the electrolytic manganese oxide, EMD) as well as drastic reduction in the amount of the expensive nickel in the structure (Ni < 0.5), without compromising its advantageous properties.In this work, the preparation and electrochemical properties of LiMn1.9Ni0.1O4 cathode materials containing very small amount of nickel (x = 0.1) and using EMD precursor have been investigated. The spinel cathode material was chosen due to its low toxicity, abundant material source and its high specific capacity of 148 mA h g−1. The commercial spinel cathode material (LiMn2O4) is a well-studied cathode system for LIB with the potential to serve as an alternative to the toxic and expensive LiCoO2. However, the main challenge with LiMn2O4 is the capacity fading due to Jahn–Teller distortion10–12 in the 3 V region,13 which is due to the generation of new phases during cycling and disproportionation reaction. In order to overcome this limitation, we have adapted a Ni-doping strategy. Literature reports have shown that doping with a small amount of Cr3+, Ni2+ and Al3+ can stabilize the spinel structure of LiMn2O4 and provides high operating voltage above 4.7 V, suppress the Jahn–Teller effect, and improve the cycling properties.14–16 Although the use of small amount of nickel in the structure (i.e., Ni < 0.5) has rarely been studied, it has been established that Ni = 0.1 provides the best electrochemistry.12 Therefore, there is a need to further explore the performance of this spinel using new low-cost synthetic routes.
Various synthetic routes have been followed to synthesize different spinel cathode materials, including solid state,17 combustion,15 co-precipitation,18 sol–gel method19 and modified pechini.20 Unfortunately these methods require elevated temperatures as high as 700–900 °C. Further, LiMn2−xNixO4 synthesized by the solid-state method is often accompanied by the formation of LixNixO impurity phases which causes capacity fading. The crystallinity of the materials is also poor and leads to the dissolution of crystal faces by an electrolyte which deteriorates the rate capability. The techniques based on the processes of co-precipitation can give single phase LiMn2−xNixO4 at lower temperatures. However, these methods involve the use of expensive reagents with complex process.21
In this work, for the first time, we opted for a low temperature aqueous reduction method22 to synthesize LiMn1.9Ni0.1O4 cathode materials. We have used NiSO4·6(H2O) as the nickel source and locally-produced low-cost EMD as the Mn source. This synthesis method not only has the advantage of using a locally-produced low-cost EMD but can also be a viable replacement to co-precipitation technique.
Experimental
Materials and preparation
Electrolytic manganese dioxide (EMD) from a South African supplier (Delta EMD Pty Ltd) and LiOH·H2O, NiSO4·6(H2O), glucose from Sigma Aldrich were used for the synthesis of spinel LiMn2−xNixO4 (x = 0 and 0.1) cathode materials.Both LiMn1.9Ni0.1O4 and its pristine material, LiMn2O4 (for comparison) were prepared using a facile and low temperature aqueous reduction synthesis method by employing electrolytic manganese dioxide (EMD), LiOH·H2O, NiSO4·6(H2O) (for the Ni-doped sample) and glucose as a reducing agent. Briefly, a stoichiometric amount of LiOH·H2O, EMD and NiSO4·6(H2O) (for the LiMn1.9Ni0.1O4 sample) was dissolved in 60 mL of double-distilled water by continuous stirring at a temperature of 80 °C. After 1 h, the appropriate amount of glucose dissolved in 20 mL of double-distilled water was added to the mixture. The stirring was continued for a further 8 h at 80 °C until the reaction was complete. The slurry was allowed to cool and settle for 12 h. After decanting, the product was washed several times with distilled water and dried at 120 °C. The resultant powder was calcined at 780 °C for 20 h in air and then cooled to room temperature naturally in the furnace. The purpose of further calcination at 780 °C to 20 h is to generate the required phase structure and composition in the LiMn2−xNixO4 product (x = 0 and 0.1). In both samples, the same method of synthesis was adopted.
Equipment and procedure
The morphology of the samples LiMn2−xNixO4 (x = 0 and 0.1) were obtained using a high resolution scanning electron microscope (JEOL, JSM-7600F), operating at an accelerating voltage of 5 kV. The EDS facility attached to the SEM gave the elemental data on the samples. The structural properties of the samples were investigated by X-ray diffraction analysis using a PANalytical X'Pert PRO PW3040/60 X-ray diffractometer with a Ni filtered Cu-Kα (λ = 0.154 nm) monochromated radiation source. Data were collected in the 2θ range of 10–90° at a scan rate of 2° min−1. The XPS data were analyzed using the XPS Peak 4.1 program.Cell fabrication and electrochemical analysis
Electrochemical cells were fabricated as follows: coin cells of 2032 were assembled using lithium metal as anode, Celgard 2400 as separator and a 1 M solution of LiPF6 in ethylene carbonate (EC), diethyl carbonate (DEC), dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume) the electrolyte. The cathode was made from a slurry using a coating procedure from a mix containing active material powder, conducting black and poly(vinylidene fluoride) binder in N-methyl-2-pyrrolidone in the proportion 80
1, by volume) the electrolyte. The cathode was made from a slurry using a coating procedure from a mix containing active material powder, conducting black and poly(vinylidene fluoride) binder in N-methyl-2-pyrrolidone in the proportion 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. The slurry was coated over aluminium foil and dried at 110 °C overnight for 12 h. The 18 mm diameter slurry-coated aluminium foil electrodes were punched out and used as cathode. Coin cells were assembled in an argon filled glove box (MBraun, Germany) with moisture and oxygen levels maintained at less than 1 ppm. The charge–discharge cycles of the cells were carried out between 3.5–4.8 V at 0.2C rate with respect to their corresponding theoretical capacities of LiMn2O4 and LiMn1.9Ni0.1O4 using a Maccor 4000 series 96-channel battery tester. The electrochemical impedance spectroscopy studies were carried out using a Bio-Logic VMP 3 Potentiostat/Galvanostat controlled by EC-Lab v10.40 software. EIS data were collected after ageing the fabricated lithium-ion cell for 24 h. Nyquist plots of the charged and discharged electrodes were recorded after allowing 1 h of stabilization.
10, respectively. The slurry was coated over aluminium foil and dried at 110 °C overnight for 12 h. The 18 mm diameter slurry-coated aluminium foil electrodes were punched out and used as cathode. Coin cells were assembled in an argon filled glove box (MBraun, Germany) with moisture and oxygen levels maintained at less than 1 ppm. The charge–discharge cycles of the cells were carried out between 3.5–4.8 V at 0.2C rate with respect to their corresponding theoretical capacities of LiMn2O4 and LiMn1.9Ni0.1O4 using a Maccor 4000 series 96-channel battery tester. The electrochemical impedance spectroscopy studies were carried out using a Bio-Logic VMP 3 Potentiostat/Galvanostat controlled by EC-Lab v10.40 software. EIS data were collected after ageing the fabricated lithium-ion cell for 24 h. Nyquist plots of the charged and discharged electrodes were recorded after allowing 1 h of stabilization.
Results and discussion
Morphological and EDS elemental analysis
Fig. 1a and b shows SEM images of LiMn2O4 and LiMn1.9Ni0.1O4 samples, respectively. The SEM image in Fig. 1b shows that the LiMn1.9Ni0.1O4 cathode materials have octahedral shape with clearly exposed (111) facets. The (111) facets are known to allow the formation of a thinner solid electrolyte interphase (SEI) than other facets thereby enhancing the Li-ion kinetics and cycling performance.23 | ||
| Fig. 1 Top-view SEM images of the products (a) LiMn2O4 and (b) LiMn1.9Ni0.1O4; particle size distributions of the cathode materials (c) LiMn2O4 and (d) LiMn1.9Ni0.1O4 from the SEM images. | ||
The estimated particle size distribution24 of the compositions LiMn2O4, and LiMn1.9Ni0.1O4 is graphically presented in Fig. 1. Fig. 1c and d indicates that the particle sizes of the cathode materials are in the range of 0.30–0.50 μm for LiMn2O4 and 0.80–1.80 μm for LiMn1.9Ni0.1O4. The calculated average particle sizes of the samples LiMn2O4 and LiMn1.9Ni0.1O4 are 0.405 and 1.332 μm, respectively.
Energy-dispersive X-ray spectroscopy (EDS) elemental analysis was carried out in order to confirm the doping of Ni-ions. Table 1 displays the EDS elemental percentage of the samples. The EDS confirms that pristine LiMn2O4 and Ni-doped LiMn1.9Ni0.1O4 spinel cathode materials were successfully synthesized using our aqueous reduction techniques. The EDS indicated that the nickel elemental quantity increases from 0.06 for pristine LiMn2O4 to 1.62 for nickel substituted LiNi0.1Mn1.9O4. The presence of carbon is due to the graphite-coating used in the SEM analysis.
| Sample | C K | O K | Mn K | Ni K | Total% |
|---|---|---|---|---|---|
| LiMn2O4 | 15.89 | 31.98 | 52.08 | 0.06 | 100 |
| LiMn1.9O4 | 8.68 | 40.80 | 48.86 | 1.62 | 100 |
Structural characterisation
The X-ray diffraction patterns to analyse the crystallographic structure and the impurity phases of the doped compounds synthesized by the aqueous reduction process are shown in Fig. 2. It is confirmed from the XRD patterns that the spinel LiMn2O4 phase (JCPDS File no. 88-1749) which indexes to a cubic spinel structure with a space group Fd3m is formed for both pristine LiMn2O4 and nickel substituted LiMn1.9Ni0.1O4 samples. The calculated lattice constants of LiMn2O4 and LiMn1.9Ni0.1O4 are 8.239 and 8.234 Å respectively. The decrease in the lattice constant of the LiMn1.9Ni0.1O4 is due to the replacement of the Mn3+ of high ionic radius (r(Mn3+) = 72.0 pm) with Ni of smaller ionic radius (62.0 pm). Also, the XRD data for the precursor EMD, LiMn2O4 and LiMn1.9Ni0.1O4 before 780 °C heat treatment are shown in Fig. 2b. The XRD peaks shifted slightly towards left with respect to EMD precursor reflection peaks, indicating structural change due to aqueous reduction reaction. | ||
| Fig. 2 X-ray diffraction patterns for the (a) LiMn2O4 and LiMn1.9Ni0.1O4 (b) EMD, LiMn2O4 and LiMn1.9Ni0.1O4 before 780 °C calcination. | ||
Fig. 3a and b shows the detailed XPS spectra of the Mn 2p3/2 peaks of the LiMn2O4, and LiMn1.9Ni0.1O4 samples, respectively. There is a broad peak width for both the materials, which indicates that the Mn exist in more than one oxidation state. The deconvoluted peaks of Mn 2p3/2 for the samples LiMn2O4 and LiMn1.9Ni0.1O4 with the obtained binding energy positions and cation distribution are summarised in Table 2. The binding energy peak positions corresponding to Mn4+ and Mn3+ are in agreement with previously reported values in the literature.25 The XPS results indicate a decrease in the Mn3+ for the Ni-doped LiMn1.9Ni0.1O4 cathode material that confirms a possible substitution of the Mn3+ by the Ni ions26,27 and results in increase in Mn valence from 3.51 of LiMn2O4 to 3.53 of LiMn1.9Ni0.1O4. This slight increase in Mn valence is needed for stabilising the spinel structure and suppressed the John–Teller distortion associated to capacity fading.27
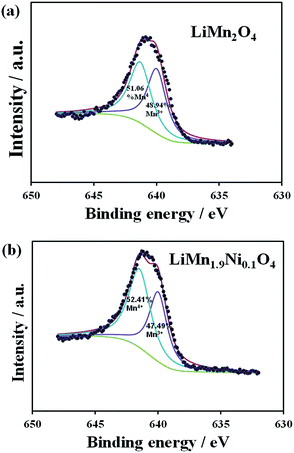 | ||
| Fig. 3 The X-ray photoelectron spectra of the (a) LiMn2O4 and (b) LiMn1.9Ni0.1O4 showing the Mn 2p3/2 peak. | ||
| Sample | Binding energy position (eV) | Cation distribution | Mn valence | ||
|---|---|---|---|---|---|
| Mn4+ | Mn3+ | Mn4+ (%) | Mn3+ (%) | ||
| LiMn2O4 | 641.3 | 639.99 | 51.06 | 48.94 | 3.51 |
| LiMn1.9Ni0.1O4 | 641.5 | 640.0 | 52.51 | 47.49 | 3.53 |
Electrochemical performance
The electrochemical activity role is played by Mn3+ in the pristine LiMn2O4. In the synthesized spinel, the Mn oxidation state is 3.5+ since an equal number of Mn3+ and Mn4+ are assumed to be present before charging. During charging, all Mn3+ convert ideally to Mn4+ by driving all Li+ ions into the anode electrode. The dissolution of manganese into the electrolyte is generated by the occurrence of the disproportion reaction 2Mn3+ (solid) → Mn4+ (solid) + Mn2+ (solution) in10,11 the 4 V region. As a result the electrochemical active Mn3+ will diminish accordingly the discharge capacity will start to fade.
The galvanostatic charge/discharge capacity performance of the cathode materials was carried out at 0.2C rates with respect to their corresponding theoretical capacities. The representative 1st, 2nd, and 40th cycle charge/discharge capacities of LiMn2−xNixO4 (x = 0 and 0.1) are displayed in Fig. 4. During the first cycle the as-synthesized cathode materials LiMn2O4, and LiMn1.9Ni0.1O4 respectively delivered discharge capacities of 137 mA h g−1 and 128 mA h g−1. The result shows that the initial discharge capacity decreases for the nickel-doped sample as expected. This trend of decrease in capacity is as a result of a reduction in the reversibly extractable Li+ ions from 1 for pristine LiMn2O4 to 1 − x for Ni-substituted lithium manganese oxides upon substitution of the electrochemically active Mn3+ ions.28 The discharge capacities of the as-synthesized cathode materials are comparable to experimentally reported values.29,30 Despite that nickel insertion into EMD is difficult, it is interesting to see that our synthesis protocol was able to insert some amount that could successfully suppress the capacity fading. The capacity contribution at high voltage 4.5–4.7 V due to Ni2+/Ni4+ is very little, most of the electrochemical capacity is at the 4.1 V due to the Mn3+/Mn4+ redox couple. In addition, the first cycle charge–discharge reversibility for the samples LiMn2O4 and LiMn1.9Ni0.1O4 is 86.9% and 69.7%, respectively. By considering the 2nd and 40th cycle capacity, it is noted that LiMn1.9Ni0.1O4 shows gain in charge–discharge reversibility of 87% and 96% at the 2nd and 40th cycle, respectively. The improvement in reversibility arises from structural stability; at the 1–2 cycles, the spinel cathode material is not properly equilibrated with the electrolyte, but this should expected to improve upon repetitive cycling, and hence the improved reversibility.
 | ||
| Fig. 5 The discharge capacity vs. cycle number for LiMn2O4 and LiMn1.9Ni0.1O4 at (a) 0.2C and (b) 0.4, 0.8, 1, 2, 3C. | ||
Next, we look at the rate capability of the two spinel materials by performing experiments at different high rates, from 0.4 to 3C (Fig. 5b). Upon completion of the rate capability experiments and the initial rate of 0.4C was repeated, LiMn1.9Ni0.1O4 lost about 7% of its initial capacity (ca. 90 vs. 84 mA h g−1) while the LiMn2O4 experienced a loss of about 16% (ca. 74 vs. 62 mA h g−1), clearly confirming the improved electrochemical stability due to the presence of the Ni in the LiMn1.9Ni0.1O4. Also, in all cases, the capacity of the LiMn1.9Ni0.1O4 is almost double to that of the LiMn2O4; for example at 1C, the capacities of the LiMn1.9Ni0.1O4 and LiMn2O4 are approximately 78 and 40 mA h g−1, respectively.
To further prove the stability of LiMn1.9Ni0.1O4, we carried out SEM analysis after 100 cycles. From the SEM images of LiMn2O4 (Fig. 6a) and LiMn1.9Ni0.1O4 (Fig. 6b) samples after 100 cycles, it is interesting to observe that the morphology of the LiMn1.9Ni0.1O4 showed a microporous but inter-connected network structures compared to the morphology of the LiMn2O4 that showed huge agglomeration of the starting nanoparticles. The morphology of the LiMn1.9Ni0.1O4 should allow for a more facile electrochemistry (in terms of stability and kinetics) than that of the agglomerated. From the above experimental findings, we can conclude that the high electrochemical performance of the LiMn1.9Ni0.1O4 over its pristine counterpart LiMn2O4 can be related to a considerable decrease in the Jahn–Teller distortion spinel.15,31
 | ||
| Fig. 7 Electrochemical impedance spectrum for LiMn2O4 and LiMn1.9Ni0.1O4 samples (a) before, (b) after 100 cycles, and inset the equivalent circuit used to interpret the impedance spectra. | ||
| R s (Ω) | R f (Ω) | CPEf (μF) | n | CPEdl (mF) | R ct (Ω) | Z w (Ω s−1/2) | ||
|---|---|---|---|---|---|---|---|---|
| Before 100 cycles | ||||||||
| LiMn2O4 | 37.04 ± 0.72 | 25.85 ± 2.32 | 15.17 ± 0.96 | 0.84 ± 0.13 | 28.37 ± 2.89 | 373.1 ± 2.68 | 42.62 ± 2.51 | |
| LiMn1.9Ni0.1O4 | 33.82 ± 0.23 | 21.51 ± 0.95 | 73.54 ± 3.26 | 0.56 ± 0.21 | 58.42 ± 8.26 | 235.8 ± 1.78 | 86.79 ± 3.57 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| After 100 cycles | ||||||||
| LiMn2O4 | 28.05 ± 1.66 | 304 ± 2.57 | 32.95 ± 2.04 | 0.77 ± 0.18 | 16.61 ± 4.56 | 1105 ± 2.34 | 17.54 ± 0.78 | |
| LiMn1.9Ni0.1O4 | 24.92 ± 3.19 | 277.9 ± 1.69 | 28.77 ± 3.6 | 0.68 ± 0.05 | 27.29 ± 9.76 | 431.8 ± 7.94 | 11.38 ± 0.57 | |
Plots of −Z′ vs. ω−1/2 for LiMn2−xNixO4 (x = 0, 0.1) is shown in Fig. 8. The diffusion coefficients for LiMn2O4 and LiMn1.9Ni0.1O4 cathode materials are 6.4 × 10−12 and 6.89 × 10−11 cm2 s−1 at room temperature, respectively. The result confirms that nickel substitution has significantly enhanced the Li+ ion diffusion, which is a magnitude higher for nickel substituted LiMn1.9Ni0.1O4 than the pristine LiMn2O4 diffusion coefficient.
Conclusions
In summary, we employed low-cost manganese precursor electrolytic manganese dioxide (EMD) and a low temperature aqueous reduction synthesis technique to successfully prepare nickel substituted spinel LiMn2−xNixO4 (x = 0 and 0.1) cathode for lithium-ion battery by using NiSO4·6H2O as nickel source. We have confirmed that the Ni-ions substituted the Mn-ions using XRD, EDS, XPS and electrochemical performance studies. The nickel-substituted sample LiMn1.9Ni0.1O4 exhibited superior capacity retention as compared to pristine LiMn2O4; LiMn1.9Ni0.1O4 retained 84% of its initial capacity whereas pristine LiMn2O4 retained only 52% of its initial capacity.The study shows that the use of a small amount of Ni to eliminate the Jahn–Teller effects of the LiMn2O4. In addition, this synthesis protocol has a great potential to be deployed for upscale-up production of pristine and Ni-doped spinel LiMn2O4 cathode materials for lithium-ion battery applications from locally-sourced and low-cost manganese precursor.
Acknowledgements
This work was supported by the CSIR. NK thanks the CSIR for PhD studentship.References
- K. Ding, J. Zhao, J. Zhou, Y. Zhao, Y. Chen, L. Liu, L. Wang, X. He and Z. Guo, Mater. Chem. Phys., 2016, 177, 31–39 CrossRef CAS.
- L. Wang, C. Yang, S. Dou, S. Wang, J. Zhang, X. Gao, J. Ma and Y. Yu, Electrochim. Acta, 2016, 219, 592–603 CrossRef CAS.
- H. Lyu, J. Liu, S. Qiu, Y. Cao, C. Hu, S. Guo and Z. Guo, J. Mater. Chem. A, 2016, 4, 9881–9889 CAS.
- S. Qiu, G. Lu, J. Liu, H. Lyu, C. Hu, B. Li, X. Yan, J. Guo and Z. Guo, RSC Adv., 2015, 5, 87286–87294 RSC.
- H. Cao, X. Wang, H. Gu, J. Liu, L. Luan, W. Liu, Y. Wang and Z. Guo, RSC Adv., 2015, 5, 34566–34571 RSC.
- R. Santhanam and B. Rambabu, J. Power Sources, 2010, 195, 5442–5451 CrossRef CAS.
- S. Patoux, L. Sannier, H. Lignier, Y. Reynier, C. Bourbon, S. Jouanneau, F. Le Cras and S. Martinet, Electrochim. Acta, 2008, 53, 4137–4145 CrossRef CAS.
- T. Ohzuku, S. Takeda and M. Iwanaga, J. Power Sources, 1999, 81, 90–94 CrossRef.
- J. M. Amarilla, R. M. Rojas and J. M. Rojo, J. Power Sources, 2011, 196, 5951–5959 CrossRef CAS.
- D. Aurbach, M. Levi, K. Gamulski, B. Markovsky, G. Salitra, E. Levi, U. Heider, L. Heider and R. Oesten, J. Power Sources, 1999, 81, 472–479 CrossRef.
- Y. Xia, Y. Zhou and M. Yoshio, J. Electrochem. Soc., 1997, 144, 2593–2600 CrossRef CAS.
- Y. Shin and A. Manthiram, J. Electrochem. Soc., 2004, 151, A204–A208 CrossRef CAS.
- B. Xu, D. Qian, Z. Wang and Y. S. Meng, Mater. Sci. Eng., R, 2012, 73, 51–65 CrossRef CAS.
- M. Aklalouch, J. M. Amarilla, R. M. Rojas, I. Saadoune and J. M. Rojo, Electrochem. Commun., 2010, 12, 548–552 CrossRef CAS.
- M. A. Kebede, N. Kunjuzwa, C. J. Jafta, M. K. Mathe and K. I. Ozoemena, Electrochim. Acta, 2014, 128, 172–177 CrossRef CAS.
- M. A. Kebede, M. J. Phasha, N. Kunjuzwa, L. J. le Roux, D. Mkhonto, K. I. Ozoemena and M. K. Mathe, Sustainable Energy Technologies and Assessments, 2014, 5, 44–49 CrossRef.
- H. Fang, Z. Wang, X. Li, H. Guo and W. Peng, Mater. Lett., 2006, 60, 1273–1275 CrossRef CAS.
- X. Qiu, X. Sun, W. Shen and N. Chen, Solid State Ionics, 1997, 93, 335–339 CrossRef CAS.
- L. H. Chi, N. N. Dinh, S. Brutti and B. Scrosati, Electrochim. Acta, 2010, 55, 5110–5116 CrossRef CAS.
- C. J. Jafta, M. K. Mathe, N. Manyala, W. D. Roos and K. I. Ozoemena, ACS Appl. Mater. Interfaces, 2013, 5, 7592–7598 CAS.
- D. Wang, I. Belharouak, G. M. Koenig, G. Zhou and K. Amine, J. Mater. Chem., 2011, 21, 9290–9295 RSC.
- V. G. Kumar, J. Gnanaraj, S. Ben-David, D. M. Pickup, E. R. Van-Eck, A. Gedanken and D. Aurbach, Chem. Mater., 2003, 15, 4211–4216 CrossRef CAS.
- B. Hai, A. K. Shukla, H. Duncan and G. Chen, J. Mater. Chem. A, 2013, 1, 759–769 CAS.
- J. Gu, X. Yang, C. Li and K. Kou, Ind. Eng. Chem. Res., 2016, 55, 10941–10946 CrossRef CAS.
- K. Raju, F. P. Nkosi, E. Viswanathan, M. K. Mathe, K. Damodaran and K. I. Ozoemena, Phys. Chem. Chem. Phys., 2016, 18, 13074–13083 RSC.
- Y. Wang, G. Yang, Z. Yang, L. Zhang, M. Fu, H. Long, Z. Li, Y. Huang and P. Lu, Electrochim. Acta, 2013, 102, 416–422 CrossRef CAS.
- X. Li, Y. Xu and C. Wang, Appl. Surf. Sci., 2009, 255, 5651–5655 CrossRef CAS.
- H. Wu, J. Tu, X. Chen, Y. Li, X. Zhao and G. Cao, J. Solid State Electrochem., 2007, 11, 173–176 CrossRef CAS.
- Y. Ito, Y. Idemoto, Y. Tsunoda and N. Koura, J. Power Sources, 2003, 119, 733–737 CrossRef.
- X. Gu, X. Li, L. Xu, H. Xu, J. Yang and Y. Qian, Int. J. Electrochem. Sci., 2012, 7, 2504–2512 CAS.
- B. Hwang, R. Santhanam and D. Liu, J. Power Sources, 2001, 97, 443–446 CrossRef.
- M. Levi and D. Aurbach, J. Phys. Chem. B, 1997, 101, 4630–4640 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



