Plasmon-mediated photothermal conversion by TiN nanocubes toward CO oxidation under solar light illumination†
Oruganti Anjaneyulua,
Satoshi Ishii*bc,
Tsubasa Imaiad,
Toyokazu Tanabee,
Shigenori Uedafg,
Tadaaki Nagao*bc and
Hideki Abe *acd
*acd
aHydrogen Production Materials Group, Center for Green Research on Energy and Environmental Materials, National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: ABE.Hideki@nims.go.jp
bWPI International Center for Material Nanoarchitectonics, National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: sishii@nims.go.jp; Nagao.Tadaaki@nims.go.jp
cCore Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
dGraduate School of Science and Technology, Saitama University, 255 Shimo-Okubo, Saitama 338-8570, Japan
eKanagawa University, 3-27 Rokkakubashi, Kanagawa-ku, Yokohama, Kanagawa 221-8686, Japan
fSynchrotron X-ray Station at SPring-8, National Institute for Materials Science, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan
gQuantum Beam Unit, National Institute for Materials Science, Sengen, Tsukuba 305-0047, Japan
First published on 14th November 2016
Abstract
Titanium-nitride (TiN) nanocubes were decorated with platinum nanoparticles via a wet-chemistry route to yield TiN-supported Pt catalysts (Pt/TiN). Under illumination of the sunlight, the Pt/TiN catalyst exhibited 100-fold higher activity than SiO2-supported Pt catalysts toward the remediation of carbon monoxide (CO) due to plasmon-mediated photothermal conversion by the TiN nanocubes.
Thermal catalysis is of the utmost importance in chemical industries such as steam methane reforming (SMR) and desulfurization of petroleum.1 Large amounts of fuels are consumed through combustions to achieve the high reaction temperatures required in thermal catalysis.1a,c,2 Most of the thermal catalysts are consisted of nanoparticles of catalytic metals being thinly dispersed and immobilized on the surface of supporting materials such as SiO2 or Al2O3 (supported catalysts: usual loadings of metal nanoparticles are 0.1–10 wt% of supports).3 In order to realize lowered reaction temperatures and reduced fuel consumption, there has been accumulated effort on the development of high-performance thermal catalysts through nanostructure- and/or compositional tailoring of supports and metal nanoparticles.4
Recently, it emerges a new class of catalysis driven not by fuel combustions but illumination of visible light (VL: wavelength = 350 to 700 nm) and/or solar light (SL), i.e., photothermal catalysis.5 Some of the materials with intense light absorption in the VL- and/or SL wavelengths region, such as carbon black, work as a photothermal medium that efficiently converts the absorbed light to heat energy.6 Such photothermal media can, when used as supporting materials, provide heat to the supported catalytic metal nanoparticles to promote desired reactions under VL- and/or SL illumination.7 Photothermal catalysis can be an ultimate solution to the challenge of fuel consumption in thermal catalysis because the sustainable SL can be utilized as the energy source instead of conventional fossil fuels. However, photothermal catalysis has been still precluded from industrial use because the carbon-based photothermal media are susceptible to the oxidative atmosphere at elevated temperatures.
Gold (Au) nanomaterials also serve as efficient supports for photothermal catalysis due to their strong plasmonic resonance in the VL- and/or SL wavelengths region (plasmon-mediated photothermal catalysis).8 The reformation reaction of ethanol and the combination reaction of hexacyano ferrate/thiosulfate were catalysed at room temperature by VL-illuminated Au nanoparticles.9 Palladium nanoparticles supported by Au nanorods (Pd/Au nanorods) efficiently catalysed Suzuki-coupling reaction under VL illumination.10 Although the Au materials are inherently tolerant to oxidative degradations, Au-based photothermal supports are not desirable for large-scale use in industry because of the materials cost and limited mineral resource. None of the earth-abundant metals such as titanium (Ti) or iron (Fe) works as efficient photothermal media for VL or SL due to their large optical losses.11
Herein, we report that titanium nitride (TiN) which consists of Ti and one of the most earth-abundant elements, nitrogen (N), can be an abundant and stable alternative to traditional photothermal media. TiN materials exhibit intense absorption in a broad optical range to generate heat under VL- and near infrared illumination.12 TiN materials are especially desirable for photothermal catalysis because of their inherent chemical stability to oxidative atmosphere. Indeed, TiN materials were used as catalyst supports in corrosive conditions for methanol electro-oxidation13 and hydrogenolysis of aryl ethers.14 We demonstrate in this report that TiN-supported platinum (Pt) nanoparticles (Pt/TiN) can significantly catalyse one of the industrially important reactions, oxidative remediation of carbon monoxide in combustion exhaust (CO oxidation: CO + 1/2O2 = CO2), under SL illumination. Thermography observation and electromagnetic simulations have elucidated that SL is efficiently absorbed and converted into heat on the TiN supports through surface-plasmon resonance, which further promotes CO oxidation over the supported Pt nanoparticles.
The desired TiN materials were synthesized by a thermal plasma method in ultra-high vacuum in a shape of nanocubes (TiN NC; see ESI† for details on the synthesis).15 The pXRD profile for the synthesized TiN NC shows peaks at 37.2, 43.3 and 63.1 degrees which correspond to the 111−, 200− and 220 reflections from a single-phased TiN with the NaCl-type structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 0.4244 nm) (Fig. 1A).16 Pt nanoparticles were deposited onto the TiN NC by chemical reduction of H2PtCl6 with NaBH4 in aqueous solution to obtain TiN-supported catalysts precisely containing the desired Pt loading (Pt/TiN). The pXRD profile for the Pt/TiN shows several peaks besides those from the TiN support at 40.0, 46.5 and 68.0 degrees, corresponding to the 111−, 200 and 220 reflections from the deposited Pt nanoparticles (Fm
m, a = 0.4244 nm) (Fig. 1A).16 Pt nanoparticles were deposited onto the TiN NC by chemical reduction of H2PtCl6 with NaBH4 in aqueous solution to obtain TiN-supported catalysts precisely containing the desired Pt loading (Pt/TiN). The pXRD profile for the Pt/TiN shows several peaks besides those from the TiN support at 40.0, 46.5 and 68.0 degrees, corresponding to the 111−, 200 and 220 reflections from the deposited Pt nanoparticles (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 0.3902 nm).17
m, a = 0.3902 nm).17
The materials were further characterized by hard-X-ray photoemission spectroscopy (HAXPES) with synchrotron radiation (photon energy = 5.95 keV; Fig. 1B and C) at SPring-8. Fig. 1B shows the HAXPES spectra for pure Ti metal, the TiN NC and Pt/TiN in the Ti 2p region. Pure metal Ti shows two photoemission peaks at 461.0 and 455.0 eV, corresponding to the Ti 2p1/2- and Ti 2p3/2 core emissions, respectively.18 Both of the Ti 2p photoemissions from the TiN NC and Pt/TiN were +1.0 eV shifted in the binding energy with respect to the corresponding photoemissions from pure metal Ti, as a result of electron transfer between electropositive Ti and electronegative N. In addition to the Ti 2p emissions from the nitride phase, there were observed less intense peaks at 456.1 and 465.0 eV for both of the TiN NC and Pt/TiN, which are assigned to oxygen-coordinated Ti4+ cations (Ti4+–O; see Fig. S1† for the HAXPES spectra in the O 1s region). These Ti4+ cations most likely existed on the very surface of the TiN NC in the forms of oxides and/or hydroxides (TiO2 or Ti(OH)4), because neither oxides nor hydroxides of Ti were recognized on either the TiN NC or Pt/TiN by the bulk-sensitive pXRD (Fig. 1).
Fig. 1C shows the N 1s emission spectra for the TiN NC and Pt/TiN. The HAXPES spectra for the TiN NC and Pt/TiN were virtually identical in both of the Ti- and N core regions, indicating that the TiN NC retained the inherent chemical state before/after the Pt deposition (see also the HAXPES spectra in the Pt 4f region in Fig. S2†). The intense N 1s peaks for both of the materials were centered at 397.4 eV, which is consistent with the reported value for TiN (Fig. 2c).13 Note that the N 1s emission from the TiN materials had a higher binding energy than those of other metallic nitrides including CrN (396.4 eV)19 and ScN (396.2 eV),20 instead, close to the binding energies of ionic nitrides such as AlN (397.3 eV)21 or Si3N4 (397.7 eV).22 These ionic nitrides are colourless because of their wide bandgaps (>3.2 eV), whereas the TiN NC is black in colour because of surface-plasmon resonance (Fig S3 and S4†).23 Band-structure calculations have demonstrated that TiN has a slightly occupied Ti 3d band above the filled N 2p band, which results in the intermediate nature between metals and ionic compounds.24,25
Fig. 2 shows transmission-electron microscope (TEM) images of the Pt/TiN. The length of each side of the TiN NC ranged from 10 to 100 nm (Fig. 2A; see also Fig. S5†). Pt nanoparticles (particle size: 5–10 nm) were uniformly deposited on to the TiN NC at Pt/Ti = 10 wt%. The surface of TiN NC was thinly coated with amorphous layers, which are likely to be oxide and/or hydroxide phases containing the Ti4+ cations (Fig. 2B; see Fig. 1B and the related discussions). High-angle annular-dark-field image (HAADF, Fig. 2C) and compositional-mapping images of the Pt/TiN (Fig. 2D–G) indicate that no alloy phase was formed at the metal/nitride interface. Note that HAXPES has also supported that Pt nanoparticles were not alloyed with the TiN supports (Fig. S2†). Compositional-mapping images of the Pt/TiN (Fig. 2D–G) also indicate that no alloy phase was formed at the metal/nitride interface.
We conducted thermal CO remediation over the Pt/TiN catalyst at 200 °C (Fig. 3A). As a control, Pt nanoparticles were deposited to spherical SiO2 nanoparticles (SiO2 NS; Aldrich, particle size = 200 nm) to obtain SiO2-supported Pt (Pt/SiO2; Pt loading weight = 10 wt%) via the same wet-chemistry route as the Pt/TiN (Fig. S6 and S7†). The Pt/SiO2 exhibited 20% of CO remediation at a duration of 46 min. The catalytic activity of the Pt/TiN NC was lower than that of the control Pt/SiO2, showing a CO remediation of 7.5% at a duration of 46 min. This may be caused by the lower Pt surface area of the Pt/TiN due to the large Pt particles, which formed a connected network structure (Fig. S7 and S8†). The TiN NC exhibited no finite activity, indicating that the TiN support is as inert as the SiO2 support toward the catalytic CO remediation.
The trend in the catalytic performance was significantly changed under SL illumination (Fig. 3B). Neither the TiN NC nor Pt/SiO2 exhibited finite CO-remediation activity at room temperature even when illuminated by a solar simulator (San-ei Electric. Co.; power density: 276 mW cm−2) with a Fresnel lens. By clear contrast to the TiN NC- or Pt/SiO2 catalysts, the Pt/TiN exhibited 8% of CO remediation at a duration of 46 min. Pt nanoparticles were active for the CO remediation only when being provided with heat energy at elevated temperatures. The TiN NC was not active for the CO remediation either at elevated temperatures or under SL illumination. In addition, the Pt/TiN was stable retaining the chemical composition and structure after the photothermal catalysis (Fig. S9†). We conclude that the TiN NC is an efficient photothermal convertor that transfers the absorbed SL energy to the supported Pt nanoparticles in the form of heat, promoting the desired CO remediation catalysis.
In order to elucidate the mechanism behind the photothermal conversion on the TiN NC, we performed electromagnetic simulations as shown in Fig. 4A and B (see also Fig. S10†). The TiN NC and SiO2 NS were assumed to be in vacuum and illuminated by monochromatic light with a wavelength of 550 nm that gives the maximum irradiance in solar spectrum. In Fig. 4A, strong absorption of the incident light is clearly visualized, which is enhanced by localized surface plasmon resonances.12 The excited surface plasmons decay into phonons, finally result in heat generation in a limited volume (plasmon-mediated photothermal conversion). The bulk- and surface temperatures of the individual TiN NC can be significantly increased by this local heat generation.
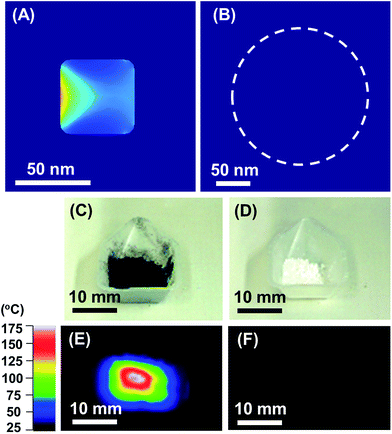
By contrast to the TiN NC, the SiO2–NS did not absorb SL to generate heat because SiO2 has a wide bandgap (<8.9 eV) to permit photoexcitation by SL (photon energy below 3.0 eV) (Fig. 4B). Indeed, powder samples of the TiN NC and the SiO2 NS are black and white respectively in colour in the daylight (Fig. 4C and D, respectively). Fig. 4E and F show the thermography images of the same powder samples of the TiN NC and SiO2 NS as Fig. 4C and D, respectively, acquired at room temperature under SL illumination. The TiN NC sample exhibited a rise in the temperature under SL illumination as a result of the plasmon-mediated photothermal conversion (Fig. 4E), whereas the temperature of the SiO2 NS sample was barely above the room temperature, 35 °C (Fig. 4F). The surface temperature of the TiN NC powder reached 175 °C, which is higher than the light-off temperature of the CO remediation over Pt catalysts.2c The Pt/TiN catalyst promoted the CO remediation under SL illumination (see Fig. 3B) because the plasmon-mediated photothermal conversion on the TiN support provides necessary heat to the catalytic center, Pt nanoparticles.
In conclusion, we have materialized photothermal catalysts consisting of Pt nanoparticles and TiN NC, Pt/TiN. The Pt/TiN catalyst exhibited enhanced catalytic activity compared with SiO2-supported Pt nanoparticles toward the CO remediation reaction under SL illumination. Electromagnetic simulations have demonstrated that the TiN NC efficiently absorbs light, converting the photon energy into heat through surface-plasmon resonances. While gold has been the primary material for the variety of photothermal applications, our result demonstrate that TiN, which is much more cheap and earth-abundant than gold, serves as a platform for the plasmon-mediated photothermal catalysis. We anticipate that TiN materials to be combined with appropriate metal co-catalysts such as Ni, and can be applied to industrial processes, achieving significant reduction in the consumption of fossil fuels by the efficient use of solar energy.
Acknowledgements
A part of this work was supported by NIMS microstructural characterization platform as a program of “Nanotechnology Platform” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The HAXPES measurements were performed under the approval of the NIMS Synchrotron X-ray Station (Proposal No. 2014B4605, 2015A4602, 2015B4602). The authors are grateful to HiSOR, Hiroshima University, and JAEA/SPring-8 for the development of HX-PES at BL15XU of SPring-8.Notes and references
- (a) O. O. James, S. Maity, M. A. Mesubi, K. O. Ogunniran, T. O. Siyanbola, S. Sahu and R. Chaubey, Green Chem., 2011, 13, 2272 RSC; (b) S. D. Davidson, H. Zhang, J. Sun and Y. Wang, Dalton Trans., 2014, 43, 11782 RSC; (c) D. Pakhare and J. Spivey, Chem. Soc. Rev., 2014, 43, 7813 RSC; (d) V. C. Srivastava, RSC Adv., 2012, 2, 759 RSC.
- (a) J. T. Kummer, J. Phys. Chem., 1986, 90, 4747 CrossRef CAS; (b) J. Kašpar, P. Fornasiero and N. Hickey, Catal. Today, 2003, 77, 419 CrossRef; (c) M. Shelef and R. W. McCabe, Catal. Today, 2000, 62, 35 CrossRef CAS; (d) H. Abe, J. Liu and K. Ariga, Mater. Today, 2016, 19, 12 CrossRef CAS; (e) M. Bowker, Chem. Soc. Rev., 2008, 37, 2204 RSC.
- (a) B. P. Bastakoti, Y. Li, N. Miyamoto, N. M. Sanchez-Ballester, H. Abe, J. Ye, P. Srinivasu and Y. Yamauchi, Chem. Commun., 2014, 50, 9101 RSC; (b) D. R. Rainer, M. Koranne, S. M. Vesecky and D. W. Goodman, J. Phys. Chem. B, 1997, 101, 10769 CrossRef CAS.
- (a) S. Mostafa, F. Behafarid, J. R. Croy, L. K. Ono, L. Li, J. C. Yang, A. I. Frenkel and B. R. Cuenya, J. Am. Chem. Soc., 2010, 132, 15714 CrossRef CAS PubMed; (b) D. Varade, H. Abe, Y. Yamauchi and K. Haraguchi, ACS Appl. Mater. Interfaces, 2013, 5, 11613 CrossRef CAS PubMed; (c) N. M. Sanchez-Ballester, G. V. Ramesh, T. Tanabe, E. Koudelkova, J. Liu, L. K. Shrestha, Y. Lvov, J. P. Hill, K. Ariga and H. Abe, J. Mater. Chem. A, 2015, 3, 6614 RSC.
- (a) D. Boyer, P. Tamarat, A. Maali, B. Lounis and M. Orrit, Science, 2002, 297, 1160 CrossRef CAS PubMed; (b) Z. J. Coppens, W. Li, D. G. Walker and J. G. Valentine, Nano Lett., 2013, 13, 1023 CrossRef CAS PubMed; (c) X. Meng, T. Wang, L. Liu, S. Ouyang, P. Li, H. Hu, T. Kako, H. Iwai, A. Tanaka and J. Ye, Angew. Chem., Int. Ed., 2014, 53, 11478 CrossRef CAS PubMed.
- (a) A.-H. Lu, X.-Q. Zhang, Q. Sun, Y. Zhang, Q. Song, F. Schüth, C. Chen and F. Cheng, Nano Res., 2016, 9, 1460 CrossRef CAS; (b) M. Zhou, S. Liu, Y. Jiang, H. Ma, M. Shi, Q. Wang, W. Zhong, W. Liao and M. M. Q. Xing, Adv. Funct. Mater., 2015, 25, 4730 CrossRef CAS.
- D. Chen, C. Wang, F. Jiang, Z. Liu, C. Shu and L.-J. Wan, J. Mater. Chem. B, 2014, 2, 4726 RSC.
- (a) H. Yang, L.-Q. He, Y.-W. Hu, X. Lu, G.-R. Li, B. Liu, B. Ren, Y. Tong and P.-P. Fang, Angew. Chem., Int. Ed., 2015, 54, 11462 CrossRef CAS PubMed; (b) H. Liu, X. Meng, T. D. Dao, H. Zhang, P. Li, K. Chang, T. Wang, M. Li, T. Nagao and J. Ye, Angew. Chem., Int. Ed., 2015, 54, 11545 CrossRef CAS PubMed.
- C.-W. Yen and M. A. El-Sayed, J. Phys. Chem. C, 2009, 113, 19585 CAS.
- F. Wang, C. Li, H. Chen, R. Jiang, L.-D. Sun, Q. Li, J. Wang, J. C. Yu and C.-H. Yan, J. Am. Chem. Soc., 2013, 135, 5588 CrossRef CAS PubMed.
- (a) P. R. West, S. Ishii, G. V. Naik, N. K. Emani, V. M. Shalaev and A. Boltasseva, Laser Photonics Rev., 2010, 4, 795 CrossRef CAS; (b) G. V. Naik, V. M. Shalaev and A. Boltasseva, Adv. Mater., 2013, 5, 3264 CrossRef PubMed.
- (a) J. C. Ndukaife, A. Mishra, U. Guler, A. G. A. Nnanna, S. T. Wereley and A. Boltasseva, ACS Nano, 2014, 8, 9035 CrossRef CAS PubMed; (b) U. Guler, S. Suslov, A. V. Kildishev, A. Boltasseva and V. M. Shalaev, Nanophotonics, 2015, 4, 269 CrossRef CAS; (c) S. Ishii, R. P. Sugavaneshwar and T. Nagao, J. Phys. Chem. C, 2016, 120, 2343 CrossRef CAS.
- M. M. Ottakam Thotiyl, T. Ravikumar and S. Sampath, J. Mater. Chem., 2010, 20, 10643 RSC.
- V. Molinari, C. Giordano, M. Antonietti and D. Esposito, J. Am. Chem. Soc., 2014, 136, 1758 CrossRef CAS PubMed.
- K. Nakamura, Earozoru Kenkyu, 2014, 29, 98 CAS.
- (a) M. M. Ottakam Thotiyl, T. Ravi Kumar and S. Sampath, J. Phys. Chem. C, 2010, 114, 17934 CrossRef; (b) W. Lengauer, J. Alloys Compd., 1992, 186, 293 CrossRef CAS; (c) M. Yang, Z. Cui and F. J. DiSalvo, Phys. Chem. Chem. Phys., 2013, 15, 1088 RSC.
- B. Fang, N. K. Chaudhari, M.-S. Kim, J. H. Kim and J.-S. Yu, J. Am. Chem. Soc., 2009, 131, 15330 CrossRef CAS PubMed.
- H. Abe, H. Yoshikawa, N. Umezawa, Y. Xu, G. Saravanan, G. V. Ramesh, T. Tanabe, R. Kodiyath, S. Ueda, N. Sekido, Y. Y. Mitarai, M. Shimoda, T. Ohno, F. Matsumoto and T. Komatsu, Phys. Chem. Chem. Phys., 2015, 17, 4879 RSC.
- O. Nishimura, K. Yabe and M. Iwaki, J. Electron Spectrosc. Relat. Phenom., 1989, 49, 335 CrossRef CAS.
- Y. M. Shulg'a, V. N. Troitskii, M. I. Aivazov and Y. G. Borodk'o, Zh. Neorg. Khim., 1976, 21, 2621 Search PubMed.
- J. A. Taylor and J. W. J. Rabalais, Chem. Phys., 1981, 75, 1735 CAS.
- L. Bois, P. L. Haridon, Y. Laurenta, X. Gouinb, P. Grangeb, J.-F. Lktard, M. Birot, J.-P. Pillot and J. Dunogub, J. Alloys Compd., 1996, 232, 244 CrossRef CAS.
- (a) J. Kim, S.-H. Jhi and K. R. Lee, J. Appl. Phys., 2011, 110, 083501 CrossRef; (b) E. Valkonen, C.-G. Ribbing and J.-E. Sundgren, Appl. Opt., 1986, 25, 3624 CrossRef CAS PubMed.
- (a) M. Kumar, S. Ishii, N. Umezawa and T. Nagao, Opt. Mater. Express, 2016, 26, 29 CrossRef; (b) M. Kumar, N. Umezawa, S. Ishii and T. Nagao, ACS Photonics, 2016, 3, 43 CrossRef CAS.
- H. Allmaier, L. Chioncel and E. Arrigoni, Phys. Rev. B, 2009, 79, 235126 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, spectral data, and TEM images. See DOI: 10.1039/c6ra22989a |
| This journal is © The Royal Society of Chemistry 2016 |