Synthesis of LaNiO3 perovskite using an EDTA-cellulose method and comparison with the conventional Pechini method: application to steam CO2 reforming of methane
Abstract
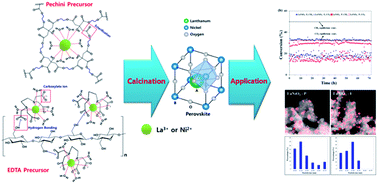
LaNiO3 type perovskite was synthesized by two different methods, and characterized by various techniques such as in situ and ex situ XRD, TPR, N2 physisorption, CO chemisorption, TGA, FT-IR, XPS, TPH, TPSR and TEM-EDS. It was found that dried Pechini and EDTA precursors had different polymerization networks, and these dissimilar bonding and coordination states of each precursor led to differences in physicochemical properties after the calcination. Thus, differences in grain size of the perovskite and textural pores, which caused different nickel particle sizes and nickel particle dispersions after the reduction, were obtained for each catalyst. The calcined catalysts were applied to steam CO2 reforming of methane. It was found that the uniform particle size distribution, smaller nickel particle size and higher contact time for LaNiO3–EDTA brought a positive effect on the catalytic activity and stability with better resistance to carbon formation for steam CO2 reforming of methane.

 Please wait while we load your content...
Please wait while we load your content...