Microbiote shift in sequencing batch reactors in response to antimicrobial ZnO nanoparticles†
Zhenghui Liu *ab,
Huifang Zhoua,
Jiefeng Liua,
Xudong Yinab,
Yufeng Maoa,
Zhisen Liub,
Zesheng Lib and
Wenyu Xieab
*ab,
Huifang Zhoua,
Jiefeng Liua,
Xudong Yinab,
Yufeng Maoa,
Zhisen Liub,
Zesheng Lib and
Wenyu Xieab
aSchool of Environmental and Biological Engineering, Guangdong University of Petrochemical Technology, Maoming, Guangdong 525000, China. E-mail: lzhhok@126.com
bTechnology Research Center for Petrochemical Resources Clean Utilization of Guangdong Province, Maoming, Guangdong 525000, China
First published on 4th November 2016
Abstract
Zinc oxide nanoparticles (ZnO NPs) have been monitored in wastewater treatment plants as their potential adverse effects on functional microorganisms have been causing increasing concern. In this study, the effects of ZnO NPs on the microbial community and the characterization of resistant/sensitive genera to ZnO NPs toxicity were investigated in sequencing batch reactors. Our results showed that ZnO NPs induced a moderate decrease in COD and nitrogen and phosphorus removal under a mid-long-term exposure. The predominant bacterial phyla were Bacteroidetes and Proteobacteria. With an increase in ZnO concentrations, the proportion of Bacteroidetes decreased, whereas that of Proteobacteria increased. Further investigations indicated that Chryseobacterium and Dechloromonas exhibit high tolerance to ZnO NPs, whereas the genera Sediminibacterium and Blvii28 exhibit specific vulnerability to ZnO NPs.
The rapid development of nanotechnology has accelerated the increasing applicability of nanoparticles (NPs) in numerous industrial products and consumer goods. NPs are particles with sizes at least one dimension between 1 and 100 nm, and more than 15% of products in the global market have been incorporated with some kind of nanoparticles via their manufacturing process.1 Moreover, the market sales for products containing nanomaterials are predicted to reach $3 trillion by 2020.2 The massive applications of nanotechnology will certainly result in the release of NPs into the environmental media, such as soil, surface water, wastewater and sewage sludge.3,4 Some types of nanoparticles, including cerium oxide NPs,5 titanium dioxide NPs,6 ZnO NPs and silver NPs,3 were also detected in the wastewater treatment plants (WWTPs), which are the major receptors of emerging pollutants and act as the last barrier before the release of effluents into the environment. The exposure to NPs in WWTPs will induce adverse effects on the bioactivity of biological treatment systems as nanoparticles can be absorbed by microorganisms.7 Nanoparticles are toxic to specific bacteria, and they negatively affect the nutrient (nitrogen and phosphorous) removal in biological treatment processes.8–10 However, the development of NPs ecotoxicology lags behind that of nanotechnology.
Because of their distinct physical and chemical properties, ZnO NPs are extensively used in semiconductors, photocatalysts, paints, coatings, cosmetics, pigments and sunscreens.11 Although Zn is a trace element for microbial growth, an excessive environmental concentration of Zn is toxic to the aquatic organisms.12,13 ZnO NPs along with nanosilver and copper oxide (CuO) NPs are regarded as biocidal NPs for bacteria, fungi and algae.14 ZnO NPs also exhibit antibacterial properties and inhibit the bacterial growth.15,16 Zheng et al. indicated that ZnO NPs can exert an acute impact on the biological processes (nitrogen and phosphorus removal) in wastewater treatment.9 Although the acute toxicity caused by ZnO NPs has been reported in recent studies,9,17,18 few studies have focused on the long-term effects of ZnO NPs on the wastewater treatment systems.19,20
Microorganisms that mediate processes in WWTPs include heterotrophic bacteria, nitrifiers, denitrifiers and polyphosphate-accumulating organisms, which are essential for the removal of nutrients and organic matter in WWTPs.21,22 Since the microbial communities are crucial for improved bioprocesses in the wastewater treatment systems, increasing concerns are focused on how the link between their structures and functionality is affected by an exposure to the entry of NPs. Denaturing gradient gel electrophoresis (DGGE) results showed that the growth of typical ammonia-oxidizing bacteria (AOB) was significantly inhibited by the high concentrations (50 mg L−1) of ZnO NPs; however, the growth of some types of denitrifying bacteria was enhanced by moderate concentrations of ZnO NPs.23 Wang et al. found that the relative abundance of nitrite-oxidizing bacteria was increased, whereas that of the ammonia oxidizing bacteria and denitrifying bacteria were not apparently changed in the membrane bioreactor (MBR) by a 454 high-throughput pyrosequencing.24 In addition, the methanogenesis Archaea to bacteria ratio was significantly reduced by the ZnO NPs in the sewage sludge anaerobic digestion systems.25 All of the abovementioned results provided information regarding the effects of ZnO oxide NPs on the microbial communities in the wastewater treatment systems. However, further in-depth investigations are required to achieve a comprehensive understanding of the microbial metabolic function and ecology. The objectives of this study were as follows: (1) to illustrate the microbial community response patterns to ZnO NPs in the reactors, and (2) to identify the most resistant and most sensitive classified genera to ZnO NPs toxicity under a mid-long term exposure.
In the present study, activated sludges in sequencing batch reactors (SBRs) were treated with various ZnO NPs concentrations for 28 days. Although there was a moderate decrease in COD and nitrogen and phosphorus removal under the mid-long term exposure to higher concentrations of ZnO NP (seen ESI Effects of ZnO NPs on the performance of SBR), no damage was observed on the activated sludge surface by SEM analysis (Fig. 1).
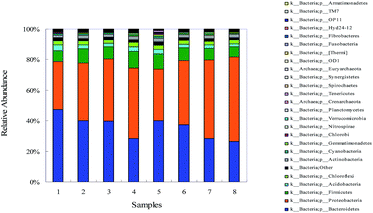
To further understand the influence of ZnO NPs on the bacterial community structures and diversity in the reactors during the exposure period, a 454 pyrosequencing was performed using an Illumina Miseq system. Bacterial diversity and distribution patterns at the phylum level for each treatment are illustrated in Fig. 2. The most predominant phylotypes at the phylum level were the members of Bacteroidetes and Proteobacteria; the sum of these two phyla represented more than 72% of the total sequences in each sample. Bacteroidetes was reported as the dominant phylum in most full-scale WWTPs, as revealed by pyrosequencing.26,27 Additionally, Proteobacteria are important for the activated sludge process performance in the WWTPs because they were observed to be responsible for the removal of organic waste, nitrogen and phosphorus.28,29 Other abundant phyla found in the samples included Firmicutes (6.81–10.66%), Acidobacteria (1.93–3.69%), Chloroflexi (2.27–2.63%), Actinobacteria (1.87–2.35%), Cyanobacteria (0.69–1.39%) and other bacteria (1.30–2.42%) (Fig. 2), and these results were partially consistent with those of the previous studies on the activated sludge communities in WWTPs of Asia.30 During the exposure period, the proportion of Bacteroidetes decreased (from 46.9% to 26.4%), whereas that of Proteobacteria increased (from 31.5% to 54.9%) with the elevated ZnO concentrations. ZnO NPs can positively affect and/or selectively inhibit the metabolic activities and growth of specific microorganisms. For instance, the growth of Firmicutes was enhanced at 1 mg L−1 ZnO NPs concentration, however, inhibited at higher ZnO NPs concentrations (Fig. 3). These results indicated that the susceptibility of the microorganisms to ZnO NPs was dependent on the type of the species.
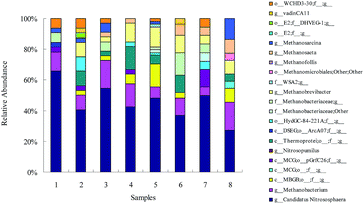
The taxonomic affiliation of archaeal sequences is illustrated in Fig. 4. Most of the sequences were affiliated to two major groups at the class level: Thaumarchaeota and Methanobacteria. All amoA-carrying members of Thaumarchaeota are regarded as microorganisms related to the autotrophic nitrification in many environments.31–33 In addition, ammonia-oxidizing archaea (AOA) belonging to the Thaumarchaeota soil group I.1b were the predominant archaea in one petroleum refinery WWTP.34 However, some studies reported that Thaumarchaeota was related to the biodegradation of the organic matter rather than the autotrophic nitrification in antibiotic spiramycin wastewater.35 Methanobacteria were common archaea existing in the wastewater of anaerobic digesters.36,37 Archaeal growth was enhanced by 69.5% at a ZnO NPs concentration of 1 mg L−1 (the proportion of 0.36% increased to 0.61%), which was mainly ascribed to the increase of Thaumarchaeota class and Marine Benthic Group B (MBGB) class. Specifically, the genus Candidatus Nitrososphaera was the most dominant archaea and was attributed to the archaeal growth under a long-term exposure at 1 mg L−1 ZnO NPs (47.2%). Additionally, Bouali et al. reported that Candidatus Nitrososphaera consists of 42% archaeal sequences in a horizontal subsurface flow constructed wetland.38 In the experiment, the proportion of Thaumarchaeota decreased when the ZnO NPs concentration was increased (Fig. 4).
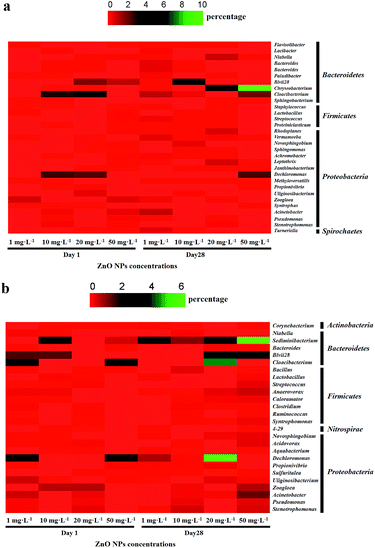
The change in the bacterial community response indicated their sensitivity and tolerance to the ZnO NPs; consequently, a heat-map including ten genera showing either the largest increase or decrease in the abundance was performed to illustrate commonalities in the microbial community changes (Fig. 5). Bacteroidetes and Proteobacteria were the two largest groups (56.6% and 40.8%, respectively) in the heat-map of ZnO resistant genera (Fig. 5a). Specifically, Chryseobacterium and Dechloromonas showed high tolerance to ZnO NPs. Other resistant genera belonged to the phyla Firmicutes and Spirochaetes. The ten most sensitive genera were affiliated to the phyla Bacteroidetes, Firmicutes and Proteobacteria (Fig. 5b) with Bacteroidetes (71.0%) and Proteobacteria (26.4%) as the most dominant groups. Two genera that belonged to the phylum Bacteroidetes, Sediminibacterium and Blvii28, showed specific vulnerability to ZnO NPs. Surprisingly, some genera, such as Acinetobacter, Dechloromonas, Cloacibacterium and Blvii28, were classified as both the most sensitive and the most resistant depending on the ZnO exposure concentrations. This observation was probably explained by the fact that NPs (including ZnO NPs) can simultaneously stimulate and inhibit the microbial-mediated processes and/or disrupt the microbial cells in the wastewater treatment systems.39 Dissecting the factors attributed to these differences, remains an important issue for future research studies.
In summary, an exposure to ZnO NPs caused a moderate decrease in COD, and nitrogen and phosphorus removal in SBR. Shifts in the microbial communities were revealed by pyrosequencing, and the characterization of the resistant/sensitive microbes was performed by the heat-map analysis. The results showed that the most dominant bacterial phyla were Bacteroidetes and Proteobacteria, and most of the archaeal sequences were affiliated to two major groups at the class level: Thaumarchaeota and Methanobacteria. The proportion of Bacteroidetes decreased at the elevated concentrations of ZnO NPs (from 46.9% to 26.4%), whereas that of Proteobacteria increased (from 31.5% to 54.9%). Chryseobacterium and Dechloromonas showed a high tolerance to ZnO NPs. Sediminibacterium and Blvii28 genera, which belong to the phylum Bacteroidetes, showed a specific vulnerability to ZnO NPs. Overall, under the mid-long term exposure to ZnO NPs, the microbial species composition shifts might provide the information for the characterization and acclimation of individual nanotolerant microorganisms in the wastewater treatment.
Acknowledgements
This research was supported by the Natural Science Foundation of Guangdong Province, China (S2013040015162, 2015A030313801), the Science and Technology Planning Project of Guangdong Province (2013B021000010, 2014A020216046), the Science and Technology Planning Project of Maoming (2014039), the Doctoral Science Foundation of Guangdong University of Petrochemical Technology (513033), and the Open Fund Project of the Technology Research Center for Petrochemical Resources Clean Utilization of Guangdong Province (201516B03).Notes and references
- N. G. Dawson, BioScience, 2008, 58, 690 CrossRef.
- M. C. Roco, J. Nanopart. Res., 2011, 13, 427–445 CrossRef.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- S. J. Klaine, P. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS PubMed.
- L. K. Limbach, R. Bereiter, E. Müller, R. Krebs, R. Gälli and W. J. Stark, Environ. Sci. Technol., 2008, 42, 5828–5833 CrossRef CAS PubMed.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Perez-Rivera and K. Hristovski, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS PubMed.
- H. Ma, P. L. Williams and S. A. Diamond, Environ. Pollut., 2013, 172, 76–85 CrossRef CAS PubMed.
- R. Sinha, R. Karan, A. Sinha and S. K. Khare, Bioresour. Technol., 2011, 102, 1516–1520 CrossRef CAS PubMed.
- X. Zheng, R. Wu and Y. Chen, Environ. Sci. Technol., 2011, 45, 2826–2832 CrossRef CAS PubMed.
- Y. Chen, H. Chen, X. Zheng and H. Mu, J. Hazard. Mater., 2012, 239–240, 88–94 CrossRef CAS PubMed.
- A. Nel, T. Xia, L. Mädler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- F. Çeçen, N. Semerci and A. G. Geyik, J. Chem. Technol. Biotechnol., 2010, 85, 520–528 Search PubMed.
- A. Cabrero, S. Fernandez, F. Mirada and J. Garcia, Water Res., 1998, 32, 1355–1362 CrossRef CAS.
- A. Ivask, O. Bondarenko, N. Jepihhina and A. Kahru, Anal. Bioanal. Chem., 2010, 398, 701–716 CrossRef CAS PubMed.
- L. K. Adams, D. Y. Lyon and P. J. J. Alvarez, Water Res., 2006, 40, 3527–3532 CrossRef CAS PubMed.
- L. Zhang, Y. Jiang, Y. Ding, M. Povey and D. York, J. Nanopart. Res., 2006, 9, 479–489 CrossRef.
- L. Hou, J. Xia, K. Li, J. Chen, X. Wu and X. Li, Water Sci. Technol., 2013, 67, 254–260 CrossRef CAS PubMed.
- F. Huang, Z. Wang, X. Mei and Z. Wu, Technol. Water Treat., 2013, 39, 14–18 CAS.
- M. Tan, G. Qiu and Y.-P. Ting, Bioresour. Technol., 2015, 185, 125–133 CrossRef CAS PubMed.
- N.-Q. Puay, G. Qiu and Y.-P. Ting, J. Cleaner Prod., 2015, 88, 139–145 CrossRef CAS.
- G. Liu, D. Wang, J. Wang and C. Mendoza, Sci. Total Environ., 2011, 409, 2852–2857 CrossRef CAS PubMed.
- Z. Liang, A. Das and Z. Hu, Water Res., 2010, 44, 5432–5438 CrossRef CAS PubMed.
- S. T. Wang, S. P. Li, W. Q. Wang and H. You, RSC Adv., 2015, 5, 67335–67342 RSC.
- Z. Wang, F. Huang, X. Mei, Q. Wang, H. Song, C. Zhu and Z. Wu, J. Membr. Sci., 2014, 471, 258–264 CrossRef CAS.
- H. Mu and Y. Chen, Water Res., 2011, 45, 5612–5620 CrossRef CAS PubMed.
- M. Hu, X. Wang, X. Wen and Y. Xia, Bioresour. Technol., 2012, 117, 72–79 CrossRef CAS PubMed.
- D. Shu, Y. He, H. Yue and Q. Wang, Bioresour. Technol., 2015, 186, 163–172 CrossRef CAS PubMed.
- C. O. Jeon, D. S. Lee and J. M. Park, Water Res., 2003, 37, 2195–2205 CrossRef CAS PubMed.
- Y. Yang, J. Quensen, J. Mathieu, Q. Wang, J. Wang, M. Li, J. M. Tiedje and P. J. J. Alvarez, Water Res., 2014, 48, 317–325 CrossRef CAS PubMed.
- T. Zhang, M.-F. Shao and L. Ye, ISME J., 2012, 6, 1137–1147 CrossRef CAS PubMed.
- M. Könneke, A. E. Bernhard, J. R. de la Torre, C. B. Walker, J. B. Waterbury and D. A. Stahl, Nature, 2005, 437, 543–546 CrossRef PubMed.
- H.-D. Park, G. F. Wells, H. Bae, C. S. Criddle and C. A. Francis, Appl. Environ. Microbiol., 2006, 72, 5643–5647 CrossRef CAS PubMed.
- T. Zhang, T. Jin, Q. Yan, M. Shao, G. Wells, C. Criddle and P. F. Hh, J. Appl. Microbiol., 2009, 107, 970–977 CrossRef CAS PubMed.
- M. Mußmann, I. Brito, A. Pitcher, J. S. Sinninghe Damsté, R. Hatzenpichler, A. Richter, J. L. Nielsen, P. H. Nielsen, A. Müller, H. Daims, M. Wagner and I. M. Head, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 16771–16776 CrossRef PubMed.
- Y. Zhang, Z. Tian, M. Liu, Z. J. Shi, L. Hale, J. Zhou and M. Yang, Environ. Sci. Technol., 2015, 49, 9124–9132 CrossRef CAS PubMed.
- M. C. Nelson, M. Morrison and Z. Yu, Bioresour. Technol., 2011, 102, 3730–3739 CrossRef CAS PubMed.
- Z. Chen, Y. Wang, K. Li and H. Zhou, J. Biosci. Bioeng., 2014, 118, 284–288 CrossRef CAS PubMed.
- M. Bouali, I. Zrafi-Nouira, A. Bakhrouf, D. Le Paslier, S. Chaussonnerie, E. Ammar and A. Sghir, Sci. Total Environ., 2012, 435, 465–471 CrossRef PubMed.
- S. Eduok, C. Hendry, R. Ferguson, B. Martin, R. Villa, B. Jefferson and F. Coulon, FEMS Microbiol. Ecol., 2015, 91, fiv082 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22823b |
| This journal is © The Royal Society of Chemistry 2016 |