A unique opportunity for the utilization of glass wastes as a resource for catalytic applications: toward a cleaner environment†
Abstract
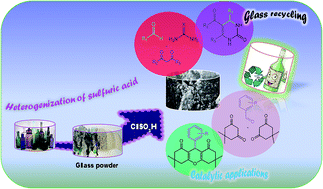
Although glass recycling has been conducted since 1970s, and even though recycled glass waste has been used in the construction of various alternative products, the utilization of glass waste for catalytic applications has not been fully considered until now. In the present work, glass waste materials were demonstrated to be accessible, convenient, and inexpensive resources as catalyst supports for the immobilization of sulfonic groups on their surfaces to produce an efficient heterogeneous solid acid nanocatalyst, the so-called nano-glass-waste-supported sulfonic acid (n-glass-waste-SO3H (n-GW-SA)). Titration and XRD, FE-SEM, EDX, FT-IR, BET, BJH, TEM, and TGA analysis techniques were employed to fully characterize the as-prepared catalyst. The glass-waste-supported sulfonic acid exhibited superior catalytic performance in multicomponent reactions (MCRs) for the one-pot synthesis of biologically useful 3,4-dihydropyrimidin-2(1H)-ones and xanthene derivatives in high to excellent yields. The eco-friendly and economic advantages of the said catalyst include its good recoverability and reusability for several runs, low price, low toxicity, and facile accessibility, manufacture, and handling.

 Please wait while we load your content...
Please wait while we load your content...