Facile enhancement of the durability and CO tolerance of CB/PtRu by poly(2,5-benzimidazole) coating via in situ polymerization†
Zehui Yang *a,
Xinxin Yua and
Fang Luob
*a,
Xinxin Yua and
Fang Luob
aSustainable Energy Laboratory, Faculty of Materials Science and Chemistry, China University of Geosciences Wuhan, 388 Lumo RD, Wuhan, 430074, China. E-mail: yeungzehui@gmail.com
bSchool of Materials and Science, Hubei University of Technology, 28 Nanli RD, Wuhan 430074, China
First published on 30th September 2016
Abstract
Here, we describe a facile method to enhance the stability of commercial CB/PtRu used in direct methanol fuel cells. In this work, 3,4-diaminobenzoic acid was in situ polymerized to form poly(2,5-benzimidazole) (ABPBI) on CB/PtRu particles. The experimental results indicated that the CO tolerance and stability of the CB/PtRu particles were enhanced when they were covered with ABPBI.
Direct methanol fuel cells (DMFCs) have been attracting considerable attention due to their being environmentally friendly and easy to transport, and due to the high energy density of methanol, and are hence recognized as promising power sources for portable devices; however, the widespread commercialization of DMFCs has been impeded by some serious problems arising on the anode side, such as carbon monoxide (CO) poisoning and the sluggish nature of the methanol oxidation reaction (MOR), as well as the low durability of the electrocatalyst leading to a decrease in fuel cell performance and lifetime of DMFC devices.1–3 Designing highly durable and CO-tolerant electrocatalysts has therefore become notably important for DMFC applications.
Alloying ruthenium (Ru) with platinum (Pt) was reported to be an efficient way to eliminate the CO poisoning problem, as Ru forms Ru(OH)ads from the partial oxidation of water under lower potential compared to Pt, which reacts with Pt(CO)ads, and in this way the activity of the catalyst recovers (Ru + H2O → Ru(OH)ads + H+ + e−, Ru(OH)ads + Pt(CO)ads → Pt + Ru + CO2 + H+ + e−).4 However, Ru dissolves in acidic medium and migrates to the cathode during the DMFC operation, resulting in deterioration in fuel cell performance and low durability.5–7 Thus, stabilizing Ru in the electrocatalyst was seriously considered in order to maintain high CO tolerance during long-term DMFC operations. There have been several reports about optimizing the weight ratio of Pt to Ru8,9 and controlling the shapes of PtRu particles10,11 to improve the durability of the catalyst, but these methods suffer from a complicated process that hinders the easy scalability of the electrocatalysts. Yang et al. coated PtRu electrocatalysts with manganese dioxide (MnO2) formed by reduction of KMnO4 and in this way successfully slowed down the dissolution of PtRu.12 Also, Yuan et al. reported that phosphomolybdic acid (PMo) successfully stabilized PtRu particles during long-term operations of DMFCs.13 Recently, nickel phosphide (Ni2P) was reported as an efficient stabilizer of PtRu by Xing et al.14 However, both of the above modifications involved complicated processes.
Aside from PtRu nanoparticles, Wei et al. recently reported a facile method to improve the durability of commercial CB/Pt (Pt deposited on carbon black (CB)) that involved carrying out in situ polymerization of aniline to form a PANI layer that stabilized the Pt nanoparticles.15 Based on the above considerations, we here describe a facile method to stabilize PtRu particles that involved an in situ polymerization of 3,4-diaminobenzoic acid to form a poly(2,5-benzimidazole) (ABPBI) layer on commercially procured CB/PtRu electrocatalysts as schematically shown in Fig. 1. The commercial CB/PtRu was first mixed with 3,4-diaminobenzoic acid (DABA), which was the monomer of ABPBI. The DABA was in situ polymerized and coated on the commercial CB/PtRu. The CO tolerance, methanol oxidation reaction, durability, and fuel cell performance of the newly fabricated electrocatalyst were systematically investigated.
 | ||
| Fig. 1 Schematic description of the preparation of CB/PtRu/ABPBI by in situ polymerization of 3,4-diaminobenzoic acid. Chemical structures of DABA and ABPBI are shown. | ||
The newly synthesized electrocatalyst was characterized using XPS to determine whether the typical elements were present in order to confirm the presence of ABPBI. As shown in Fig. 2a, a peak at 400 eV was observed in the XPS spectrum of the as-synthesized electrocatalyst after vigorous washing with DMAc (to remove the monomer) but was not observed in the spectrum of the commercial CB/PtRu, suggesting the successful polymerization of ABPBI. The narrow scan (Fig. 2b) showed this peak to be sharp, and it was assigned to pyrrolic nitrogen from ABPBI.16 Meanwhile, the typical Pt4f and Ru3p peaks were observed in spectra of 75 eV and 463.5 eV, as shown in Fig. 2c and d. In addition, the weight residue was decreased from 62.3 wt% to 55.8 wt% after polymerization, as shown in Fig. 2e, due to the additional ABPBI in the electrocatalyst, which was calculated to be 10.4 wt% based on a constant weight ratio between CB and PtRu. Thus, the weight percents of CB, Pt, Ru and ABPBI in CB/PtRu/ABPBI were 33.8 wt%, 37.2 wt%, 18.6 wt% and 10.4 wt%, respectively, which were slightly different from those of the commercial CB/PtRu (CB, 40 wt%, Pt: 40 wt%, Ru: 20 wt%). The XPS and TGA results indicated that the ABPBI successfully covered the CB/PtRu. Based on the density of ABPBI (1.27 g cm−3)17 and surface area of Vulcan XC-72R (235 m2 g−1),5 the thickness of the ABPBI layer was calculated to be ∼1 nm. We have reported that such a thin polymer layer negligibly affected the electronic conductivity of carbon-supporting materials.18,19
The electrochemical surface area (ECSA) was evaluated based on the equation ECSA = QH/(210 × Pt loading amount),20,21 where QH is the charge of the hydrogen electro-adsorption peak from 0.02 V to 0.3 V vs. RHE in the cyclic voltammetry curve, as shown in Fig. 3. The initial ECSA of the newly fabricated CB/PtRu/ABPBI was 59.6 m2 gPt−1, which was decreased by 16% after polymerization on CB/PtRu (71.1 m2 gPt−1). The decrease in ECSA indicated that the coverage of ABPBI on CB/PtRu slightly affected the hydrogen adsorption/desorption since the ABPBI layer was only 1 nm thick, and only 16% of the PtRu active sites were covered by ABPBI. The long-term durability of electrocatalyst was tested by carrying out cycling of the potential between 0.6 V and 1.0 V vs. RHE to check the stability of PtRu (see ESI, Fig. S1†). As can be seen in Fig. 3a, CB/PtRu showed a large drop in ECSA after the initial 600 cycles, which was attributed to the dissolution of Ru in the electrolyte since Ru can easily dissolve in acidic medium. After 4200 cycles, the retention of ECSA was only 50%; in contrast, CB/PtRu/ABPBI showed stable hydrogen adsorption/desorption peaks (Fig. 3b) and only lost 12% of the initial ECSA after 4200 cycles (Fig. 3c), suggesting that CB/PtRu/ABPBI was more durable than commercial CB/PtRu due to the coverage of ABPBI slowing down the Ru dissolution and Pt aggregation. Moreover, after the durability test, the ECSA of CB/PtRu/ABPBI was 52.0 m2 gPt−1, which was 1.5 times higher than that of CB/PtRu (35.6 m2 gPt−1), as shown in Fig. 3d. After the durability test, the electrocatalysts were collected by sonication in isopropanol for TEM imaging. As shown in Fig. 4, the particle size of CB/PtRu increased from 3.2 ± 0.2 nm before the durability test to 6.1 ± 2.7 nm after the test, while that of CB/PtRu/ABPBI only increased from 3.3 ± 0.2 nm to 4.0 ± 0.6 nm (for histograms, see ESI, Fig. S2†), indicating that the presence of the ABPBI layer on CB/PtRu was important to enhance the durability of the electrocatalyst. It should be noted that the particle size showed almost negligible growth after polymerization of ABPBI.
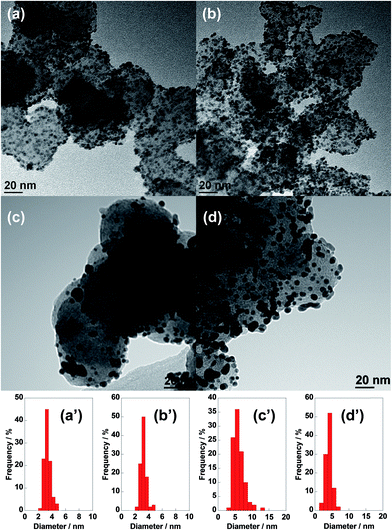 | ||
| Fig. 4 TEM images of CB/PtRu (left) and CB/PtRu/ABPBI (right) before (top) and after (bottom) the durability test. Relative frequency histograms of the PtRu nanoparticle size distributions are shown. | ||
As is well known, CO poisoning is one of the main problems for the widespread commercialization of DMFC since the CO species generated from the incomplete methanol oxidation make Pt inactive during the fuel cell operation, resulting in degradation in performance and durability. CO stripping is a typical measurement to evaluate the CO tolerance of the electrocatalyst. As shown in Fig. 5a, the CO oxidation peak of CB/PtRu was observed at 679 mV vs. RHE before the durability test. After coating with ABPBI, the CO oxidation peak slightly shifted to a negative position and was found at 670 mV vs. RHE as shown in Fig. 5b, indicating that the adsorbed CO species became more readily oxidized on CB/PtRu/ABPBI. After the durability test, the CO oxidation peak prominently shifted to 1065 mV vs. RHE for commercial CB/PtRu, which was comparable to that of CB/Pt (Fig. S2†), suggesting that almost all the Ru dissolved during the cycling of the potential since Ru is important for CO tolerance. Interestingly, the CO oxidation peak of CB/PtRu/ABPBI (691 mV) showed only a 21 mV shift after the durability test, which was 18 times lower than that of CB/PtRu. The greater stability of the CO oxidation peak of CB/PtRu/ABPBI than that of CB/PtRu was due to the lower level of dissolution of Ru, which was stabilized by ABPBI during the potential cycling, as confirmed by the XPS measurements of the two electrocatalysts after the durability test. The Ru3p peak of commercial CB/PtRu disappeared after the durability test, while the Ru3p and N1s peaks were still observed for CB/PtRu/ABPBI after the durability test, as shown in Fig. S3.† The stabilization of Ru by ABPBI was attributed to the electron delocalization between the Ru d orbitals and ABPBI π-conjugated ligands and partial ionization due to the electron transfer from Ru to ABPBI.22,23 The electron delocalization changed the electronic structure of Ru, impeding the dissolution of Ru. Also, as seen in Fig. 5c and d, the onset potential of the CO oxidation of CB/PtRu/ABPBI was lower than that of CB/PtRu both before and after the durability tests. This observation implied that the introduction of ABPBI to the electrocatalyst facilitated the CO oxidation and this easier oxidation would have been due to the nitrogen on ABPBI lowering the strength of the adsorption of CO onto PtRu particles to a level similar to that of the adsorption of CO onto phosphorus.24–26
The methanol oxidation reaction (MOR) is the anodic reaction in the real DMFC. Thus, the activities of the two electrocatalysts were measured in N2-saturated 0.5 M H2SO4 with an additional 1 M methanol in the electrolyte. As seen in Fig. 6, the mass current density of CB/PtRu slightly decreased from 0.8 A mgPt−1 to 0.74 A mgPt−1 after being coated with ABPBI due to the blocking of the Pt active sites and of the adsorption of methanol. The magnitude of this decrease was similar to that of the ECSA. The obtained mass current density of CB/PtRu suggested that methanol was still adsorbed and oxidized on PtRu nanoparticles even when 16% of the PtRu active sites were covered by ABPBI. The mass current density of CB/PtRu/ABPBI was 86% of its initial value after the durability test. In sharp contrast, the mass current density of CB/PtRu decreased by 45% after 4200 potential cycles because of the lower durability of CB/PtRu and increase in size of PtRu nanoparticles resulting from the durability test. Thus, the ABPBI layer contributed to the higher activity after the durability test. From MOR curves, the CO tolerance can also be evaluated from the ratio of If to Ib, which represent the anodic and reverse anodic peaks, respectively. The higher If/Ib ratio suggests a higher CO tolerance because the Ib peak represents the oxidation of Pt(CO)ads which generates in the If peak.27 Surprisingly, the If/Ib ratio of CB/PtRu/ABPBI was almost twice that of CB/PtRu before the durability test. This higher CO tolerance may have been due to the stabilization of Pt(OH)ads species by ABPBI since Pt–OH–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) and Pt(OH)ads are essential for the elimination of CO species from Pt surfaces.28 The higher If/Ib ratio of CB/PtRu/APBI showed that CB/PtRu/ABPBI was more tolerant towards CO than was CB/PtRu, which was consistent with the CO stripping results. After the durability test, the If/Ib ratio of CB/PtRu sharply decreased to 1.62 due to the dissolution of Ru during the cycling of the potential. The If/Ib ratio of commercial CB/PtRu was comparable to that of the commercial CB/Pt (Fig. S4†) indicated that almost all the Ru dissolved. As shown in Table 1, the If/Ib ratio of CB/PtRu/ABPBI was 4.54, i.e., 2.8 times higher that of CB/PtRu, with the considerable increase due to the protection of Ru by the ABPBI coating. The ABPBI layer was essential for slowing down the Ru dissolution.
and Pt(OH)ads are essential for the elimination of CO species from Pt surfaces.28 The higher If/Ib ratio of CB/PtRu/APBI showed that CB/PtRu/ABPBI was more tolerant towards CO than was CB/PtRu, which was consistent with the CO stripping results. After the durability test, the If/Ib ratio of CB/PtRu sharply decreased to 1.62 due to the dissolution of Ru during the cycling of the potential. The If/Ib ratio of commercial CB/PtRu was comparable to that of the commercial CB/Pt (Fig. S4†) indicated that almost all the Ru dissolved. As shown in Table 1, the If/Ib ratio of CB/PtRu/ABPBI was 4.54, i.e., 2.8 times higher that of CB/PtRu, with the considerable increase due to the protection of Ru by the ABPBI coating. The ABPBI layer was essential for slowing down the Ru dissolution.
 | ||
| Fig. 6 Methanol oxidation reaction measured in N2-saturated 0.5 M H2SO4 and 1 M CH3OH of (a) CB/PtRu and (b) CB/PtRu/ABPBI before (solid line) and after (dotted line) the durability test. | ||
| Electrocatalyst | Pre-test mass current (A mgPt−1) | Post-test mass current (A mgPt−1) | Pre-test If/Ib | Post-test If/Ib |
|---|---|---|---|---|
| CB/PtRu | 0.80 | 0.44 | 2.76 | 1.62 |
| CB/PtRu/ABPBI | 0.74 | 0.64 | 5.06 | 4.54 |
The fuel cell performances of two membrane electrode assemblies (MEAs) were tested at 60 °C with 100% relative humidity. As shown in Fig. 7, the maximum power density of CB/PtRu/ABPBI was 73 mW cm−2, which was slightly higher than that of commercial CB/PtRu (63.5 mW cm−2). These comparable power density values suggested that the ABPBI coverage negligibly affected the fuel cell performance and electronic conductivity of carbon black, yet enhanced the CO tolerance and durability.
 | ||
| Fig. 7 I–V polarization and power density curves of MEAs fabricated from commercial CB/PtRu (black line) and CB/PtRu/ABPBI (red line) at 60 °C with 100% relative humidity. | ||
In summary, the ABPBI was successfully made to cover CB/PtRu via an in situ polymerization. CB/PtRu/ABPBI lost only 16% of its initial ECSA and maintained almost the same CO tolerance after 4200 cycles of the potential between 0.6 V and 1.0 V vs. RHE. In contrast, CB/PtRu lost 50% of its ECSA and CO tolerance after the durability test, apparently due to the absence of ABPBI. The ABPBI layer was important for preventing the dissolution of Ru and the aggregation of Pt, resulting in an enhancement in durability and CO tolerance. The results we obtained by using this facile method provide useful information for the design and fabrication of durable and CO-tolerant anodic electrocatalysts for use in DMFCs. The effects of the thickness of ABPBI on the durability and CO tolerance are still being investigated in our laboratory.
Acknowledgements
Z. Yang gratefully acknowledges support of a start-up grant from China University of Geosciences Wuhan (CUG).Notes and references
- H. Huang and X. Wang, J. Mater. Chem. A, 2014, 2, 6266–6291 CAS
.
- C. Koenigsmann and S. S. Wong, Energy Environ. Sci., 2011, 4, 1161–1176 CAS
.
- X. Zhao, M. Yin, L. Ma, L. Liang, C. Liu, J. Liao, T. Lu and W. Xing, Energy Environ. Sci., 2011, 4, 2736–2753 CAS
.
- V. Selvaraj and M. Alagar, Electrochem. Commun., 2007, 9, 1145–1153 CrossRef CAS
.
- Z. Yang, C. Kim, S. Hirata, T. Fujigaya and N. Nakashima, ACS Appl. Mater. Interfaces, 2015, 7, 15885–15891 CAS
.
- Z. Yang, M. R. Berber and N. Nakashima, J. Mater. Chem. A, 2014, 2, 18875–18880 CAS
.
- H. Gharibi, M. Amani, H. Pahlavanzadeh and M. Kazemeini, Electrochim. Acta, 2013, 97, 216–225 CrossRef CAS
.
- E. V. Spinacé, A. O. Neto, T. R. R. Vasconcelos and M. Linardi, J. Power Sources, 2004, 137, 17–23 CrossRef
.
- S.-H. Liu, W.-Y. Yu, C.-H. Chen, A.-Y. Lo, B.-J. Hwang, S.-H. Chien and S.-B. Liu, Chem. Mater., 2008, 20, 1622–1628 CrossRef CAS
.
- C. Yang, M. Zhou and L. Gao, ACS Appl. Mater. Interfaces, 2014, 6, 18938–18950 CAS
.
- Y. Cheng, P. K. Shen, M. Saunders and S. P. Jiang, Electrochim. Acta, 2015, 177, 217–226 CrossRef CAS
.
- C. Zhou, F. Peng, H. Wang, H. Yu, C. Peng and J. Yang, Electrochem. Commun., 2010, 12, 1210–1213 CrossRef CAS
.
- X. Jin, B. He, J. Miao, J. Yuan, Q. Zhang and L. Niu, Carbon, 2012, 50, 3083–3091 CrossRef CAS
.
- J. Chang, L. Feng, C. Liu and W. Xing, ChemSusChem, 2015, 8, 3340–3347 CrossRef CAS PubMed
.
- S. Chen, Z. Wei, X. Qi, L. Dong, Y. G. Guo, L. Wan, Z. Shao and L. Li, J. Am. Chem. Soc., 2012, 134, 13252–13255 CrossRef CAS PubMed
.
- W. Ding, Z. Wei, S. Chen, X. Qi, T. Yang, J. Hu, D. Wang, L.-J. Wan, S. F. Alvi and L. Li, Angew. Chem., 2013, 125, 11971–11975 CrossRef
.
- S. C. Kumbharkar, P. B. Karadkar and U. K. Kharul, J. Membr. Sci., 2006, 286, 161–169 CrossRef CAS
.
- Z. Yang, I. Moriguchi and N. Nakashima, ACS Appl. Mater. Interfaces, 2015, 7, 9800–9806 CAS
.
- Z. Yang, T. Fujigaya and N. Nakashima, J. Mater. Chem. A, 2015, 3, 14318–14324 CAS
.
- Z. Yang and N. Nakashima, Sci. Rep., 2015, 5, 12236 CrossRef CAS PubMed
.
- D. Wang, H. L. Xin, R. Hovden, H. Wang, Y. Yu, D. A. Muller, F. J. Disalvo and H. D. Abruña, Nat. Mater., 2013, 12, 81–87 CrossRef CAS PubMed
.
- P. C. Ford, D. P. Rudd, R. Gaunder and H. Taube, J. Am. Chem. Soc., 1968, 90, 1187–1194 CrossRef CAS
.
- S. I. Gorelsky, A. B. P. Lever and M. Ebadi, Coord. Chem. Rev., 2002, 230, 97–105 CrossRef CAS
.
- L.-X. Ding, A.-L. Wang, G.-R. Li, Z.-Q. Liu, W.-X. Zhao, C.-Y. Su and Y.-X. Tong, J. Am. Chem. Soc., 2012, 134, 5730–5733 CrossRef CAS PubMed
.
- X. Xue, J. Ge, C. Liu, W. Xing and T. Lu, Electrochem. Commun., 2006, 8, 1280–1286 CrossRef CAS
.
- G. Yang, Y. Chen, Y. Zhou, Y. Tang and T. Lu, Electrochem. Commun., 2010, 12, 492–495 CrossRef CAS
.
- Z. Yang, I. H. Hafez, M. R. Berber and N. Nakashima, ChemCatChem, 2015, 7, 808–813 CrossRef CAS
.
- T. Fujigaya, M. Okamoto, K. Matsumoto, K. Kaneko and N. Nakashima, ChemCatChem, 2013, 5, 1701–1704 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22775a |
| This journal is © The Royal Society of Chemistry 2016 |



