Facile synthesis of methyl propylaminopropanoate functionalized magnetic nanoparticles for removal of acid red 114 from aqueous solution
Abstract
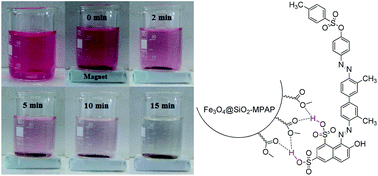
Methyl propylaminopropanoate-coated Fe3O4@SiO2 nanoparticles (Fe3O4@SiO2–MPAP NPs) was prepared as a novel adsorbent and used for the removal of acid red 114 (AR-114) from aqueous solution through hydrogen bonding interactions between the sulfonate groups of the dye and the ester carbonyl groups of the adsorbent. Characterization of the obtained new adsorbent was achieved by FT-IR, XRD and TEM. According to the experimental results, about 95.8% of AR-114 was removed from aqueous solution at the adsorbent amount of 0.24 g L−1 at pH = 2 in 60 min. The adsorption kinetics and isotherm results demonstrate that the kinetics and equilibrium adsorptions can be well-described by the pseudo-second-order kinetics and Langmuir models, respectively. Thermodynamics studies depicted that the adsorption of hazardous dye AR-114 was spontaneous and endothermic with randomness of the process. Furthermore, the Fe3O4@SiO2–MPAP NPs could be simply recovered by an external magnet and it exhibited striking recyclability and reusability for five cycles. Such functional nanoparticles are appropriate adsorbents for removal of dyes containing sulfonate groups from aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...