Modeling and experimental study on the solubility and mass transfer of CO2 into aqueous DEA solution using a stirrer bubble column
Abstract
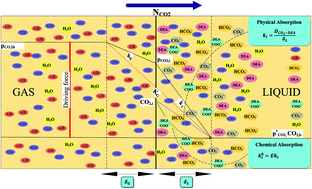
In this research, the chemical absorption rate and solubility of carbon dioxide into DEA aqueous solutions were investigated in a stirrer bubble column. Experiments envelopment the molarity of DEA, CO2 partial pressures and stirrer speed are 0.2–2.0 M, 300–450 kPa and 0–600 rpm, respectively. The DEA and CO2 concentration and gas–liquid flow rate effects on the mass transfer performance were measured in terms of CO2 capture efficiency, loading, mass transfer flux and mass transfer coefficient. A thermodynamic model, according to the Pitzer's GE model is provided for the equilibrium solubility of CO2 in DEA aqueous solutions. The results have shown that the CO2 loading in range of 0.16–0.40 and mass transfer rate in range of 0.36 × 10−5 to 0.97 × 10−5 kmol m−2 s−1 increases with DEA concentration and CO2 partial pressure. Also the results indicate that the quantity of kl increases with addition of DEA concentration, liquid and gas flow rate. Film parameter increases with increasing of DEA concentration and decreases with increasing of CO2 partial pressure. Absorption percent of CO2 was varied in range of 30–57%. The maximum absorption rate of CO2 at partial pressure of 45 kPa has been obtaining 1.8 g min−1 when the DEA concentration is 2.0 M.

 Please wait while we load your content...
Please wait while we load your content...