Stabilizing polysulfide-shuttle in a Li–S battery using transition metal carbide nanostructures†
Abstract
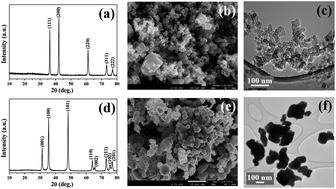
Stabilizing ‘polysulfide-shuttle’ is one of the key bottlenecks that hinders the practical application of high energy density Li–S batteries. Traditional strategies to physically/chemically confine polysulfides within the pores of carbonaceous materials have resulted in limited success. In search of alternative mechanisms, herein, we present an intrinsic adsorption strategy to address both cycle life and rate capability issues in Li–S batteries. Combining experimental studies and spin-polarized Density Functional Theory (DFT) calculations, we have shown that nanostructured transition metal carbides (TMC) such as TiC and WC can adsorb polysulfides thereby enhancing the polysulfides' redox process. The combination of preferential surface adsorption of intermediate polysulfides and the reversible binding properties of lower order polysulfides on sulfiphilic metal carbide surfaces has resulted in a reversible capacity of 860 mA h g−1 and stability over 100 charge/discharge cycles. Thus, nanostructured metal carbides with their exceptional adsorption capability towards dissolved polysulfides, could be an alternative electrode candidate for Li–S batteries.

 Please wait while we load your content...
Please wait while we load your content...