Hyper-crosslinked cyclodextrin porous polymer: an efficient CO2 capturing material with tunable porosity†‡
Bo Meng§
a,
Haiying Li§bc,
Shannon M. Mahurin*c,
Honglai Liu*b and
Sheng Dai*ac
aDepartment of Chemistry, University of Tennessee, Knoxville, TN 37996, USA. E-mail: dais@ornl.gov
bState Key Laboratory of Chemical Engineering and Department of Chemistry, East China University of Science and Technology, Shanghai, 200237, China. E-mail: hlliu@ecust.edu.cn
cChemical Sciences Division, Oak Ridge National Laboratory, 1 Bethel Valley Road, Oak Ridge, TN 37831, USA. E-mail: mahurinsm@ornl.gov
First published on 11th November 2016
Abstract
We designed and synthesized cyclodextrin (CD)-based hyper-crosslinked porous polymers (HCPPs) for selective CO2 adsorption and storage. We also explored the effect of monomer size on micropore formation, and determined a feasible way to tailor the porosity of the materials during the hyper-crosslinking process.
As a representative form of microporous organic polymers (MOPs), hyper-crosslinked polymers (HCPs) have been regarded as promising materials for a variety of applications because of unique properties such as permanent microporosity, physicochemical stability, and facile preparation.1–3 Since their initial introduction, these porous materials have been applied in many diverse fields including gas storage and separation,4,5 catalysis,6,7 pollutant absorption,8,9 and as conducting polymers.10 The microporosity of HCPs is usually generated from extensive crosslinking of monomers (sometimes with the assistance of external hyper-crosslinkers), where at least two chemical bonds are connected to the framework. The crosslinked structures make the networks highly rigid and unlikely to collapse,11 resulting in HCP materials with small pore size, high surface area and large micropore volume. In order to satisfy the demands of widely varying applications, the properties of HCPs, particularly with respect to the surface morphology, intrinsic porosity and chemical functionality, usually must be adjusted and optimized accordingly. This is generally accomplished by varying the monomer structure, incorporated functionality and synthetic conditions.12,13 Therefore, the development of novel HCPs that possess advanced properties specifically tailored for practical applications, as well as the correlation of structural variability in the building monomers to the final material properties, are of interest from both applied and theoretical perspectives.
As an abundant and inexpensive biomass, carbohydrates provide a rich and plentiful source of material. Carbohydrates are usually formed in rigid pyranose ring structures and composed of several stereoselective hydroxyl groups. These provide a molecular basis for the structural variations, which has been demonstrated to be critical in determining materials properties.14 Recently, we reported the preparation of a hyper-crosslinked β-cyclodextrin (βCD) porous polymer (BnCD-HCPP), and its application to both the adsorption of aromatic compounds and as a support for catalysis promotion.15 Cyclodextrins are cyclic oligosaccharides, with the most common types composed of six, seven, or eight glucose units linked through α-(1,4)-glucosidic bonds (termed α-, β-, γ-cyclodextrin, respectively). They form torus-like structures with a hydrophobic cavity inside, which enables the formation of host–guest inclusion complexes with suitably sized molecules.16 This property was the basis of our CD-based porous polymer design, and was reinforced via the facile Friedel–Crafts alkylation after CD benzylation. The resulting BnCD-HCPP possesses high BET surface area and pore volume. Analysis of the pore volume distribution revealed the formation of predominately micropores during the hyper-crosslinking process, though some meso- and macropores were also created.15 Though the presence of micropores are often more desirable because of enhanced performance, especially in applications such as selective gas adsorption and storage,17 little progress has been made in controlling the pore size distribution during hyper-crosslinking using the Friedel–Crafts alkylation route.18 The multiple reactive sites on the monomer result in a random hyper-crosslinking process, which makes it unpredictable and difficult to control.
Therefore, we focus on the application of CD-based HCPPs for selective CO2 adsorption and storage. Four CD-based HCPPs were synthesized and evaluated, with either αCD or βCD incorporated. In order to further enhance the selective CO2 adsorption performance, we specifically designed αBnCD6OH-HCPP and βBnCD6OH-HCPP, which contain a free hydroxyl at the C-6 position. The affinity of the hydroxyl group at the C-6 position of the carbohydrate to CO2 has been previously described.14,19 Furthermore, we also compared the microporosities of the αCD-based HCPPs and βCD-based HCPPs, to explore the relationship between the monomer cavities and the resulting porosity. The α-CD has a slightly smaller cavity (5.7–13.7 Å) than β-CD (7.8–15.3 Å), but the influence of this small size difference on the microporosity generated in the final material has not been explored.20
Four benzylated CDs were synthesized according to the diagrams shown in Scheme 1 and were subsequently used as the monomers for the construction of the HCPs. The syntheses of αBnCD and βBnCD were achieved through the application of the typical benzylation procedure. The cyclodextrin was dissolved in dry DMF at 0 °C, and NaH was added portionwise. After stirring for 15 min, benzyl bromide was injected slowly, and the reaction system was kept stirring overnight at room temperature. The reaction mixture was then quenched by methanol, concentrated, extracted by methylene chloride, and finally purified by silica gel chromatography for a total yield of approximately 80%. The structures of the α/βBnCDs were confirmed by both 1H nuclear magnetic resonance (NMR) and 13C NMR (see ESI‡). The peaks at 7.0 ppm in the 1H NMR and the peaks at 130 ppm in 13C NMR correspond to the three substituted phenyl groups on C2, C3, and C6, while the remaining peaks represent the carbohydrate backbone and the methylene group on the benzyl group. Since it has been demonstrated that the hydroxyl group at the C-6 position of a carbohydrate can interact with the CO2 molecule either through intermolecular interactions or carbonate formation, we specifically synthesized αBnCD6OH and βBnCD6OH monomers with 6-OH free, in order to further improve selective CO2 adsorption. The prepared α/βBnCDs were treated with acetic anhydride and TMSOTf at −40 °C, respectively, to allow the benzyl group at the C-6 position to be transformed into an acetyl group. Then the acetyl group was subsequently removed by sodium methoxide to give the α/βBnCD6OH. The integration of the proton peaks in 1H NMR as well as the disappearance of the methylene peak at C-6, demonstrate the successful removal of the benzyl group at C-6. The structures of these compounds were also confirmed by MALDI-TOFMS (see ESI†).
 | ||
| Scheme 1 Synthetic strategy of cyclodextrin monomers and corresponding hyper-crosslinked cyclodextrin-based porous polymers. | ||
The synthesized monomers were subsequently hyper-crosslinked to obtain the corresponding HCPP via Friedel–Crafts oxidative coupling polymerization with formaldehyde diethyl acetal (FDA) upon the activation of ferric chloride (FeCl3) in anhydrous dichloroethane. After refluxing at 80 °C for 24 h, the resulting brown polymer was washed by methanol and water three times, purified by Soxhlet extraction with methanol, and dried in vacuum at 60 °C for 24 h. The structures of the BnCD-HCPPs were verified by solid-state 13C CP/MAS NMR (Fig. S1‡). The resonance peaks around 130 ppm and the peaks between 30–40 ppm can be assigned to the aromatic carbons and linkers. The peaks at 50–80 ppm correspond to the skeleton of the cyclodextrin backbone in the polymer. The thermal stability of the prepared HCPPs was investigated by thermal gravimetric analysis (TGA) (Fig. S2‡). The traces of these four materials are very similar with the curves of αBnCD-HCPP, αBnCD6OH-HCPP and βBnCD-HCPP being nearly identical. The βBnCD6OH-HCPP curve is also similar in shape to the other three though the decomposition temperature is slightly lower which could be due to fewer linkages in the polymer. Generally, these materials display excellent thermal stability compared with other CD-based materials, with decomposition temperature up to 300 °C and 60–70 wt% mass remaining even at 800 °C.
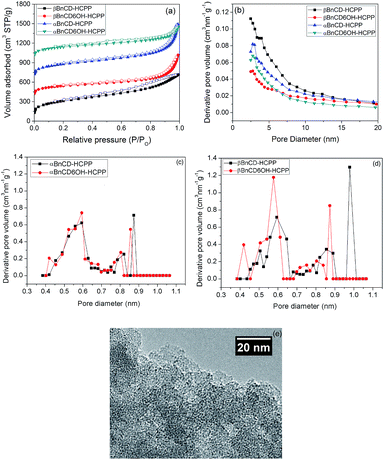
The textural properties of the CD-based HCPPs were measured by nitrogen adsorption analysis at 77 K. As shown in Fig. 1, the αBnCD-HCPP, αBnCD6OH-HCPP, and βBnCD6OH-HCPP materials exhibit a combination of type I and type II isotherms with high nitrogen uptake at low pressure and a continuous adsorption at high pressure. All of these materials show both microporosity and mesoporosity in the CD-based HCPPs which could be generated from the irregular stacking of the rigid and torqued CD molecules as well as from the original cavities inside the CDs. In addition, the βBnCD-HCPP exhibits a non-reversible adsorption isotherm with the appearance of hysteresis in the desorption curve which seldom occurs in HCPPs. Basically, this kind of type H4 isotherm loop is often associated with narrow slit-like pores, yet the type I isotherm character still indicates the presence of microporosity in the material.21 Based on the adsorption isotherms, specific surface areas were calculated using Brunauer–Emmett–Teller (BET) theory. The four HCPPs exhibited relatively high BET surface areas ranging from 871 m2 g−1 to 1225 m2 g−1, where βBnCD-HCPP possessed the highest value. This can be attributed to its larger monomer size and greater number of benzyl substituents in the monomer unit available for hyper-crosslinking. The pore size distributions of the HCPPs were also calculated and are displayed in Fig. 1b. There is a strong increase in the distribution at low diameter confirming that micropores were predominately generated with only a small fraction of meso- and macropores. To further investigate the pore size, we used carbon dioxide adsorption at 273 K to measure the pore size distribution (see Fig. 1c and d). It is noteworthy that αCD-based HCPPs show more concentrated micropores than the corresponding βCD-based HCPPs. A clear diminution can be observed near the pore diameter of 1 nm, which demonstrates the feasibility of controlling the micropore distribution by manipulating the cyclodextrin cavity size.
Because of the high surface area and large fraction of micropores, the CD-based materials have potential as CO2 sorbents in carbon capture applications. Consequently, the ability of the CD-based HCPPs to selectively adsorb CO2 was investigated with adsorption isotherms measured up to 1 bar at 273 K and 298 K (Fig. 2). At 1 bar, these materials exhibited perfectly reversible isotherms with a CO2 adsorption capacity as high as 10.77 wt% (2.45 mmol g−1) at 273 K and 6.20 wt% (1.41 mmol g−1) at 298 K, which compares well with other hypercrosslinked polymers.22 The βBnCD-HCPP shows higher CO2 adsorption ability than βBnCD6OH-HCPP at both temperatures, while the CO2 adsorption capacity of αBnCD-HCPP and αBnCD6OH-HCPP is essentially the same. The fully benzylated CDs possess more benzene rings that can participate in the hyper-crosslinking process, which contributes to the formation of higher BET surface area, and enables enhanced CO2 adsorption capability. In order to investigate the interaction of CO2 with the CD-based sorbents, we calculated the heats of adsorption for the HCPPs using a variant of the Clausius–Clapeyron equation where the adsorption isotherms were fit to a dual Langmuir model. From Table 1, the heats of adsorption were fairly similar ranging from 25.9 to 27.8 kJ mol−1, where βBnCD6OH-HCPP exhibited the highest adsorption energy. These values indicate that the CO2 undergoes strong physisorption rather than a chemisorptive process. The adsorption energy is comparable to values for porous polycyclotrimers23 reported to be ∼26–27 kJ mol−1 but lower than amine-functionalized porous polymers at ∼50 kJ mol−1 that chemically bind CO2.24
 | ||
| Fig. 2 CO2 and N2 (solid symbols), adsorption–desorption isotherms (open symbols) of HCPPs (a–d) up to 1.01 bar at 273 K and 298 K and the heats of adsorption (e). | ||
| HCPPs | Surface areaa (SBET)/m2 g−1 | Micropore surface areab (Smicro)/m2 g−1 | CO2 uptakec/mmol g−1 (wt%) | CO2/N2 selectivity (initial slope, 273 K/298 K) | Qst/kJ mol−1 | |
|---|---|---|---|---|---|---|
| 273 K | 298 K | |||||
| a Surface area calculated from nitrogen adsorption isotherms at 77 K by BET equation.b Calculated by T-method.c Measured at the pressure of 1 bar. | ||||||
| βBnCD-HCPP | 1225 | 224 | 2.45 (10.77) | 1.41 (6.20) | 26/18 | 25.9 |
| βBnCD6OH-HCPP | 880 | 445 | 2.01 (8.86) | 1.17 (5.16) | 47/30 | 27.8 |
| αBnCD-HCPP | 989 | 315 | 2.38 (10.49) | 1.38 (6.09) | 35/38 | 27.0 |
| αBnCD6OH-HCPP | 871 | 377 | 2.32 (10.23) | 1.36 (5.97) | 41/32 | 27.3 |
Furthermore, the CO2/N2 adsorption selectivity of these materials was also measured so as to evaluate their industrial capability. The selectivity was calculated based on the ratio of the slope of the CO2 and N2 isotherms at low pressure (i.e., in the Henry's law region). The selectivity of the materials ranges from 26 to 47 at 273 K, and from 18 to 38 at 298 K (Table 1, Fig. S3‡). Except for the αBnCD-HCPP sample at 298 K, the BnCD6OH-HCPPs show higher CO2/N2 selectivity compared to the fully benzylated BnCD-HCPPs, which demonstrates the effectiveness of the free hydroxyl group to enhance CO2 adsorption at low loading. Moreover, the CO2/N2 adsorption selectivity of αBnCD-HCPP at 298 K is essentially equal to that at 273 K, but slightly higher than the corresponding αBnCD6OH-HCPP at 298 K. It indicates that αCD-based HCPPs are less likely to be influenced by the temperature variation, and suggests their robust performance in CO2 selective adsorption.
In summary, we have designed and synthesized four CD-based HCPPs through a facile and efficient hyper-crosslinking route. Compared to more established microporous materials used in CO2 capture, the CD-based HCPPs are more environmentally friendly, and exhibit sufficient CO2 adsorption capacities, which are comparable or even higher than other hydroxyl-functionalized materials. The BET surface area of these HCPPs is up to 1225 m2 g−1, which is ascribed to micropores generated during the hyper-crosslinking process and the original internal cavities of the cyclodextrin building blocks. The HCPPs also exhibit good CO2 adsorption capacity (10.77 wt%, 273 K and 1 bar) and CO2/N2 adsorption selectivity (47 at 273 K). Moreover, the CO2/N2 adsorption selectivity of αCD based HCPPs are less likely to be influenced by temperature variation than βCD-based HCPPs, and specifically, αBnCD-HCPP shows a steady CO2/N2 adsorption selectivity under different temperatures. In addition, we also demonstrate that a small decrease in the monomer from βCD to αCD can lead to an enhancement in the micropores in the resulting HCPPs, which indicates that the monomer size can have a profound effect on pore formation and further can be used as a means to tailor the porosity of the materials during the hyper-crosslinking process.
Acknowledgements
H. Li and H. Liu thank the National Key Technologies R&D Program (2015BAC04B01), the National Natural Science Foundation of China (No. 91334203), and the 111 Project of China (No. B08021). Work by S. Mahurin and S. Dai was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences (BES), Chemical Sciences, Geosciences, and Biosciences Division.Notes and references
- R. Dawson, A. I. Cooper and D. J. Adams, Prog. Polym. Sci., 2012, 37, 530–563 CrossRef CAS.
- S. J. Xu, Y. L. Luo and B. E. Tan, Macromol. Rapid Commun., 2013, 34, 471–484 CrossRef CAS PubMed.
- L. X. Tan and B. E. Tan, Acta Chim. Sin., 2015, 73, 530–540 CrossRef CAS.
- Y. Han, L. M. Zhang, Y. C. Zhao, T. Wang and B. H. Han, ACS Appl. Mater. Interfaces, 2013, 5, 4166–4172 CAS.
- Y. L. Luo, B. Y. Li, W. Wang, K. B. Wu and B. Tan, Adv. Mater., 2012, 24, 5703–5707 CrossRef CAS PubMed.
- W. K. An, M. Y. Han, C. A. Wang, S. M. Yu, Y. Zhang, S. Bai and W. Wang, Chem.–Eur. J., 2014, 20, 11019–11028 CrossRef CAS PubMed.
- S. Bhunia, B. Banerjee and A. Bhaumik, Chem. Commun., 2015, 51, 5020–5023 RSC.
- L. Xiang, Y. L. Zhu, S. Gu, D. Y. Chen, X. Fu, Y. D. Zhang, G. P. Yu, C. Y. Pan and Y. H. Hu, Macromol. Rapid Commun., 2015, 36, 1566–1571 CrossRef CAS PubMed.
- K. Schute and M. Rose, ChemSusChem, 2015, 8, 3419–3423 CrossRef CAS PubMed.
- A. I. Cooper, Adv. Mater., 2009, 21, 1291–1295 CrossRef CAS.
- N. Fontanals, R. M. Marce, F. Borrull and P. A. G. Cormack, Polym. Chem., 2015, 6, 7231–7244 RSC.
- L. J. Abbott and C. M. Colina, Macromolecules, 2014, 47, 5409–5415 CrossRef CAS.
- S. Bonakala and S. Balasubramanian, J. Phys. Chem. B, 2016, 120, 557–565 CrossRef CAS PubMed.
- H. Li, B. Meng, S. M. Mahurin, S. H. Chai, K. M. Nelson, D. C. Baker, H. L. Liu and S. Dai, J. Mater. Chem. A, 2015, 3, 20913–20918 CAS.
- H. Li, B. Meng, S. H. Chai, H. L. Liu and S. Dai, Chem. Sci., 2016, 7, 905–909 RSC.
- W. C. E. Schofield, C. D. Bain and J. P. S. Badyal, Chem. Mater., 2012, 24, 1645–1653 CrossRef CAS.
- G. Y. Li, B. A. Zhang, J. Yan and Z. G. Wang, Chem. Commun., 2016, 52, 1143–1146 RSC.
- M. Seo, S. Kim, J. Oh, S. J. Kim and M. A. Hillmyer, J. Am. Chem. Soc., 2015, 137, 600–603 CrossRef CAS PubMed.
- J. J. Gassensmith, H. Furukawa, R. A. Smaldone, R. S. Forgan, Y. Y. Botros, O. M. Yaghi and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 15312–15315 CrossRef CAS PubMed.
- T. Ratvijitvech, M. Barrow, A. I. Cooper and D. J. Adams, Polym. Chem., 2015, 6, 7280–7285 RSC.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- R. Dawson, A. I. Cooper and D. J. Adams, Polym. Int., 2013, 62, 345–352 CrossRef CAS.
- A. Zukal, E. Slovakova, H. Balcar and J. Sedlacek, Macromol. Chem. Phys., 2013, 214, 2016–2026 CrossRef CAS.
- V. Guillerm, L. J. Weselinski, M. Alkordi, M. I. H. Mohideen, Y. Belmabkhout, A. J. Cairns and M. Eddaoudi, Chem. Commun., 2014, 50, 1937–1940 RSC.
Footnotes |
| † This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan). |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18307g |
| § These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |