DOI:
10.1039/C6RA22430J
(Communication)
RSC Adv., 2016,
6, 95314-95319
Constructing densely functionalized Hajos–Parrish-type ketones with six contiguous stereogenic centers and two quaternary carbons in a formal [2 + 2 + 2] cycloaddition cascade†
Received
7th September 2016
, Accepted 23rd September 2016
First published on 26th September 2016
Abstract
A [2 + 2 + 2] annulation of 2-methylcyclopentane-1,3-dione and nitrostyrenes has been achieved via a cascade double Michael–Henry reaction that provides densely functionalized Hajos–Parrish-type ketones with two quaternary carbons and six contiguous stereogenic centers. An uncommon and intriguing example of the high self-disproportionation of enantiomers (SDE) in the crystallization of racemic 3i was observed. The spontaneous high resolution of racemic mixture of 3i into its enantiopure forms via regular crystallization without the need to utilize a chiral resolving agent or external stimuli has achieved. The structures of the appropriate derivatives, (±)-3a, (±)-5a, (±)-3f, and (−)-3i, were further confirmed by their single-crystal X-ray crystallographic analyses.
Introduction
The widely known Hajos–Parrish ketone (HPK)1 prepared from the Hajos–Parrish–Eder–Sauer–Wiechert reaction (HPESW reaction)2 has been generally utilized in the total synthesis of naturally occurring compounds and biologically active molecules, especially terpenoids and steroids (Fig. 1).3 For example, Shair and coworkers completed the total synthesis of cortistatin A beginning from a Hajos–Parrish ketone,4 and Deslongchamps and coworkers achieved the total synthesis of ouabagenin starting from a Hajos–Parrish ketone in a 41-step transformation.5 In addition to these applications in total synthesis, the study of the synthesis of highly functionalized HPK derivatives, e.g.,“iso-Hajos–Parrish” ketones,6 continues to be of great interest. However, despite these developments, a multicomponent domino reaction employing 2-methylcyclopentane-1,3-dione for the construction of highly functionalized HPK derivatives, especially those with nitro substitutents,7 known as a “synthetic chameleon” functional group, has rarely been attained.8 On the other hand, to our knowledge, the cascade reaction of 2-alkylcyclopentane-1,3-dione with two equivalents of nitroalkene9 via a formal [2 + 2 + 2] annulation,10 providing the synthesis of highly functionalized HPK analogues has not been revealed (Scheme 1).11 Such a unique domino reaction is a fascinating theme of discovery. With the stated background and with the goal to broaden our investigations12,13 of multicomponent organocatalytic asymmetric annulations14 via domino reactions15 or one-pot syntheses,16 we were prompted to examine the practicality of the double Michael–Henry cascade reaction of 2-methylcyclopentane-1,3-dione (1), and two equivalents of nitroalkene (2), Scheme 1. Herein, we report the details of our exploratory results, which afford high diastereoselectivity for the structurally distinct class of highly functionalized HPK (3) containing six contiguous stereogenic centers and two quaternary stereocenters.17
 |
| | Fig. 1 Selected illustrations of the synthesis of natural occurring compounds employing the Hajos–Parrish ketone and its analogues. | |
 |
| | Scheme 1 [2 + 2 + 2] annulation transformation of HPK derivative 3. | |
Results and discussion
The extremely low solubility of 2-methylcyclopentane-1,3-dione (1) in most organic solvents was troublesome for the reaction; after many attempts, CH3CN was observed to be the best solvent medium for assessing the reaction, although 1 in CH3CN was still poorly soluble.18
Initially, a solution of 1 and 3.5 equivalents of nitrostyrene 2a in CH3CN was treated with Jørgensen–Hayashi catalyst I–HOAc (20 mol%), with no observation of the formation of product 3a, Table 1, entry 1. The same outcomes were observed in the reactions with catalyst I with an excess of HOAc (150 mol%) or DIPEA (20 mol%), Table 1, entries 2 and 3. The reactions with pyrrolidine (II, 150 mol%) or prolinol (III, 20 mol%) in CH3CN or CH3CN–H2O were also in vain, Table 1, entries 4 and 5. Therefore, we were delighted to observe that the reaction with Takemoto catalyst (IV) and DIPEA (20 mol%) for 70 h afforded an 20% yield of the product 3a, Table 1, entry 6. Treatment with DIPEA (20 mol%) and the other bifunctional chiral thiourea derivatives, e.g. V, VI, gave lesser yields, 22% and 15%, respectively (table entries 7 and 8). Surprisingly, the product 3a obtained in the above reactions had no enantioselectivity (∼0% ee). This outcome suggested that the reaction may be facilitated by DIPEA, but not by the organocatalyst. This hypothesis was strengthened by the fact that the same reaction with an excess amount of DIPEA (150 mol%) in the absence of catalysts gave 64% yield of 3a in 12 h, Table 1, entry 9. However, the reaction with a catalytic amount of DIPEA (20 mol%) only provided 23% yield of adduct 3a, Table 1, entry 10. Although the solubility of the starting compounds was a pivotal issue, the reaction with an excess amount of DIPEA (150 mol%) in water gave no sign of reaction after 18 h (Table 1, entry 11). Other attempts to conduct the reaction in CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were fruitless and we observed only trace amounts of product 3a, Table 1, entry 12–16. The reaction conditions of DIPEA (150 mol%) at 0.2 M scale of 1 (Table 1, entry 9) were the best ones since the reactions at diluted conditions provided lower yields, (Table 1, entries 17–19). The reaction of two equivalents of 1 and one equivalent of 2a with DIPEA (80 mol%) in CH3CN for 2 h afforded 22% yield of the intermediate 4a (Table 1, entry 20). Notably, 4a was unstable and it gradually decomposed back to 1 and 2a after standing at room temperature for a few days.
2) were fruitless and we observed only trace amounts of product 3a, Table 1, entry 12–16. The reaction conditions of DIPEA (150 mol%) at 0.2 M scale of 1 (Table 1, entry 9) were the best ones since the reactions at diluted conditions provided lower yields, (Table 1, entries 17–19). The reaction of two equivalents of 1 and one equivalent of 2a with DIPEA (80 mol%) in CH3CN for 2 h afforded 22% yield of the intermediate 4a (Table 1, entry 20). Notably, 4a was unstable and it gradually decomposed back to 1 and 2a after standing at room temperature for a few days.
Table 1 A screening of the catalysts, additives, solvents, and other reaction conditions for the cascade reactionsa
After establishing the optimal reaction conditions (Table 1, entry 9), we next inspected the cascade reaction with a series of nitrostyrenes 2. The results were promising, generally with good stereoselectivities (Table 2). Most reactions were completed within one day to afford adducts 3 and 5 as the only isolable products along with the highly insoluble polymer solids, which arose from the self-polymerization of nitrostyrenes 2 with DIPEA. The reaction with an electron donating substituent (e.g., methyl) on the benzene ring of nitrostyrenes, 2b, gave a lesser yield of 3b (41%), and afforded much lower yield of 3c (20%) in the reaction with a much stronger electron donating substituent (e.g., methoxy) on the benzene ring of nitrostyrenes, 2c (Table 2, entries 2 and 3). Much more polymerized insoluble solids were observed in the above two reactions. The reaction of 1a with the 4-halo substituted nitrostyrenes 2d–2f afforded 60 to 46% yields of adduct 3d–3f (Table 2, entries 4–6). Interestingly, the reaction with 2-chloro substituted nitrostyrene, 2g, provided an excellent transformation and gave 81% yield of 3g, Table 2, entry 7. The reaction of the 4-nitro substituted nitrostyrene, 2h, with an electron withdrawing substituent, gave a good yield of 3h (77%), while the 2-nitro substituted nitrostyrene, 2i, gave 38% yield of 3i and 30% yield of 5i (Table 2, entries 8 and 9). The structures of 3a, 5a, 3f, and 3i were unequivocally determined by their single crystal X-ray crystallographic analyses (Fig. 2). It is interesting to note that the high self-disproportionation of enantiomers (SDE) effect19,20 occurred during the crystallization process of racemic 3i. A chiral crystal of (−)-3i was obtained and its absolute stereochemistry was unequivocally established by its X-ray crystallographic analysis.21 Specifically, a sample of 15 mg of racemic 3i, prepared as described above, was dissolved with CH2Cl2 (15 mL) in a vial, followed by the slow addition of hexane (2 mL) down the inside of the vial and the two-layer solution was maintained throughout the addition process. The solution was capped and left to stand at room temperature for slow evaporation over 5 days to give some crystals (ca. 100 crystals) of the compound 3i. A crystal was subjected for the single crystal X-ray analysis (as shown in the Fig. S4 and Table S4, see ESI†). Surprisingly, a chiral space group: orthorhombic P212121 was observed in the crystal! In order to understand the sign of its specific rotation and to probe the approximate frequency of occurrence of the SDE effect in the recrystallization process, another two crystals from the same batch were arbitrarily selected and subjected for single crystal X-ray analysis. Notably, each of them had the same chiral space group, orthorhombic P212121, with the same absolute configuration as the previous crystal. The two single crystal X-ray analyses are shown in the Fig. S5, Table S5, Fig. S6, and Table S6, see ESI.† On the other hand, another batch of ca. 40 mg of racemic 3i was subject to the same recrystallization process as described previously to give ca. 150 crystals. From them, 46 crystals were arbitrarily selected and individually subjected to the HPLC analysis with a chiral column (Chiralpack IC). Eluent: 25% THF–hexane, flow rate: 1.0 mL min−1. The two enantiomers were separated at Rt 5.6 min and Rt 8.0 min. For the 46 crystals analyzed, 16 of them eluted with Rt 5.6 min (>99% ee), 14 of them eluted with Rt 8.0 min (>99% ee), 2 of them displayed >80% ee, 4 of them were almost a racemate, and 10 of them had low ee. The pure enantiomer was individually collected, checking with the aid of the HPLC analysis, and subjected to optical rotation analysis. For Rt 5.6 min: [α]29D +10.2 (c 0.58, acetone); for Rt 8.0 min: [α]29D −9.1 (c 0.36, acetone).22 In addition, it has been suggested that compounds qualified for forming H-bonding would be exceptionally inclined to display a significant SDE effect in achiral chromatography separations. In this context, a SDE test was executed on a racemic mixture of 3i, but no SDE (Δee ≈ 0%) was detected in the fractions obtained from the achiral silica-gel chromatography. Furthermore, the chiral HPLC analysis of the arbitrarily selected six crystals from the crystallization of racemic 3a showed no SDE (Δee ≈ 0%). After the historical and special demonstration of tweezer-separation of sodium ammonium tartrate crystals by Pasteur in the 19th century, the search for a convenient and high resolution protocol continues to be a pivotal subject in scientific studies. However, rare examples have been revealed regarding the high resolution of racemic mixtures into their enantiopure forms via regular crystallization without the need to utilize a chiral resolving agent or external stimuli. Furthermore, even with the advances of some technologies, the quest for enhanced chiral resolution methods is a long way from being complete, and this challenge remains to be conquered. As a result, the high magnitude of SDE in the crystallization process of racemic 3i is an unusual and intriguing example. The observation of a high SDE effect in the crystallization of racemic 3i not only yielded an enantiopure product,23 but also established a new vista for potential application of this process in the resolution of various types of compounds,24 and also may help to further our understanding of the origin of chirality in nature.25 Since straightforward slow evaporation of the racemic 3i solution afforded self-resolution in crystalline form and provided substantial amounts of the enantiopure 3i in crystal form, it is highly reasonable to speculate that in the prebiotic era certain chemicals could also undergo a similar process to yield enantiopure substances followed by the subsequent asymmetric catalysis (amplification reaction) and produced the status quo with the surviving enantiopure natural products.26
Table 2 Examples of cascade double Michael–Henry reactiona

|
| Entry |
Ar |
Products |
t (h) |
Yield of 3b (%) |
dr (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)c 5)c |
| Unless otherwise noted, the reactions were conducted on a 0.2 M scale of 1 (1.0 equiv.) and 2a (3.5 equiv.) in CH3CN at rt. Yields of 3 isolated. Determined by 1H NMR of the crude reaction mixture. 38% yield of 3i and 30% yield of 5i. |
| 1 |
Ph |
3a, 5a |
12 |
64 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 2 |
4-Me-Ph |
3b, 5b |
15 |
41 |
84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 3 |
4-MeO-Ph |
3c, 5c |
20 |
20 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 4 |
4-F-Ph |
3d, 5d |
15 |
60 |
88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 5 |
4-Cl-Ph |
3e, 5e |
14 |
56 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 6 |
4-Br-Ph |
3f, 5f |
20 |
46 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 7 |
2-Cl-Ph |
3g, 5g |
20 |
81 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 8 |
4-NO2-Ph |
3h, 5h |
24 |
77 |
81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 9 |
2-NO2-Ph |
3i, 5i |
15 |
38 (30)d |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
 |
| | Fig. 2 Stereo plots of the X-ray crystal structures of (±)-3a, (±)-5a, (±)-3f and (−)-3i: C, gray; O, red; N, blue; Br, purple. | |
To account for the stereochemical outcome of this transformation, we proposed a conceivable mechanism (Scheme 2). The initial conjugate addition of 1 on nitrostyrene 2a, activated by DIPEA, followed by the subsequent conjugate addition of the intermediate A to nitrostyrene 2a via TS B, and the subsequent Henry reaction via TS C would give the adduct 3a as the major isomer.27 It is noteworthy that reasonable amounts of the polymer residue, which is highly insoluble in most of the organic solvents, were observed in these reactions. This residue arose from the DIPEA-triggered polymerization of nitrostyrene since the same residue was observed in the reaction in the absence of dione 1.
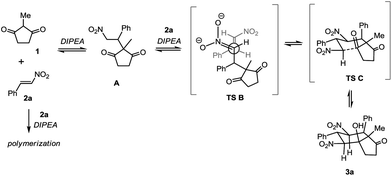 |
| | Scheme 2 Proposed reaction mechanism. | |
Conclusions
In summary, a [2 + 2 + 2] annulation of 2-methylcyclopentane-1,3-dione and nitrostyrenes has been achieved via a cascade double Michael–Henry reaction that provides densely functionalized Hajos–Parrish type ketones with six contiguous stereogenic centers, including two quaternary carbons. DIPEA was identified as the best reagent for accelerating the reaction and provided diastereoselectivities under benign reaction conditions. The exquisite execution of the cascade reaction represents a straightforward and efficient approach to synthesize highly functionalized Hajos–Parrish type ketones, which ought to find broad applications in organic synthesis (for examples of the compounds with the methyloctahydro-1H-indenol skeleton, (Fig. 3). The structures of the appropriate derivatives, (±)-3a, (±)-5a, (±)-3f, and (−)-3i, were individually confirmed by single-crystal X-ray crystallographic analyses. In addition, we succeeded in demonstrating, in an unusual and intriguing example, a spontaneous high resolution of a racemic mixture into its enantiopure forms via regular crystallization without the need to utilize a chiral resolving agent or external stimuli. The observation of a high SDE effect in the crystallization of the racemic mixture of 3i not only afforded an enantiopure product, but also pointed toward the potential application of this process in the resolution of various types of compounds, and in addition, may extend our understanding of the origin of chirality in nature.
 |
| | Fig. 3 Examples of naturally occurring compounds or biologically active agents with a methyloctahydro-1H-indenol skeleton. | |
Conflict of interest
The authors declare no competing financial interest.
Acknowledgements
We acknowledge the financial support for this study from the Ministry of Science and Technology (MOST, Taiwan) and thank the instrument center of MOST for analyses of compounds.
Notes and references
-
(a) P. Wieland and K. Miescher, Helv. Chim. Acta, 1950, 33, 2215 CrossRef CAS;
(b) A. J. Birch, J. A. K. Quartey and H. Smith, J. Chem. Soc., 1952, 1768 RSC;
(c) Z. G. Hajos, D. R. Parrish and E. P. Oliveto, Tetrahedron, 1968, 24, 2039 CrossRef CAS PubMed;
(d) U. Eder, G. Sauer and R. Wiechert, Angew. Chem., 1971, 83, 492 CrossRef;
(e) Z. G. Hajos and D. R. Parrish, J. Org. Chem., 1974, 39, 1615 CrossRef CAS;
(f) Z. G. Hajos and D. R. Parrish, Org. Synth., 1985, 63, 26 CrossRef CAS;
(g) P. Zhou, L. Zhang, S. Luo and J.-P. Cheng, J. Org. Chem., 2012, 77, 2526 CrossRef CAS PubMed;
(h) C. Xu, L. Zhang, P. Zhou, S. Luo and J.-P. Cheng, Synthesis, 2013, 45, 1939 CrossRef CAS.
-
(a) Z. G. Hajos and D. R. Parrish, Ger. Pat., DE 2102623, 1971;
(b) U. Eder, G. R. Sauer and R. Wiechert, Angew. Chem., Int. Ed., 1971, 10, 496 CrossRef CAS;
(c) Z. G. Hajos and D. R. Parrish, J. Org. Chem., 1974, 39, 1615 CrossRef CAS. For recent reviews:
(d) Comprehensive Organic Name Reactions and Reagents, ed. Z. Wang, John Wiley & Sons, Inc, 2010, ch. 290, pp. 1305–1309 Search PubMed. For recent examples:
(e) S. G. Davies, R. L. Sheppard, A. D. Smith and J. E. Thomson, Chem. Commun., 2005, 3802 RSC;
(f) G. A. Rance and A. N. Khlobystov, Nanoscale, 2014, 6, 11141 RSC;
(g) S. G. Davies, A. J. Russell, R. L. Sheppard, A. D. Smith and J. E. Thomson, Org. Biomol. Chem., 2007, 5, 3190 RSC;
(h) P. H.-Y. Cheong, K. N. Houk, J. S. Warrier and S. Hanessian, Adv. Synth. Catal., 2004, 346, 1111 CrossRef CAS.
-
(a) D. T. Hog, F. M. E. Huber, G. Jimnez-Oss, P. Mayer, K. N. Houk and D. Trauner, Chem.–Eur. J., 2015, 21, 13646 CrossRef CAS PubMed;
(b) Y. Tang, J.-T. Liu, P. Chen, M.-C. Lv, Z.-Z. Wang and Y.-K. Huang, J. Org. Chem., 2014, 79, 11729 CrossRef CAS PubMed;
(c) S. Yamashita, K. Iso, K. Kitajima, M. Himuro and M. Hirama, J. Org. Chem., 2011, 76, 2408 CrossRef CAS PubMed;
(d) G. A. Molander, M. S. Quirmbach, L. F. Silva Jr, K. C. Spencer and J. Balsells, Org. Lett., 2001, 3, 2257 CrossRef CAS PubMed;
(e) D. Renneberg, H. Pfander and C. J. Leumann, J. Org. Chem., 2000, 65, 9069 CrossRef CAS PubMed;
(f) L. A. Paquette, T.-Z. Wang and M. R. Sivik, J. Am. Chem. Soc., 1994, 116, 11323 CrossRef CAS;
(g) T. Sugimura and L. A. Paquette, J. Am. Chem. Soc., 1987, 109, 3017 CrossRef CAS;
(h) S. Qian and G. Zhao, Chem. Commun., 2012, 48, 3530 RSC;
(i) T. J. Reddy, G. Bordeau and L. Trimble, Org. Lett., 2006, 8, 5585 CrossRef CAS PubMed;
(j) O. V. Larionov and E. J. Corey, J. Am. Chem. Soc., 2008, 130, 2954 CrossRef CAS PubMed;
(k) C. Zeng, C. Zheng, J. Zhao and G. Zhao, Org. Lett., 2013, 15, 5846 CrossRef CAS PubMed.
- H. M. Lee, C. Nieto-Oberhuber and M. D. Shair, J. Am. Chem. Soc., 2008, 130, 16864 CrossRef CAS PubMed.
- H. Zhang, M. S. Reddy, S. Phoenix and P. Deslongchamps, Angew. Chem., Int. Ed., 2008, 47, 1272 CrossRef CAS PubMed.
- J. M. Eagan, M. Hori, J. Wu, K. S. Kanyiva and S. A. Snyder, Angew. Chem., Int. Ed., 2015, 54, 7842 CrossRef CAS PubMed.
- For a recent review of the application of nitro compound in organocatalysis, see:
(a) L. S. Aitken, N. R. Arezki, A. Dell'Isola and A. J. A. Cobb, Synthesis, 2013, 45, 2627 CrossRef CAS. For a recent review of naturally occurring nitro compounds, see:
(b) R. Parry, S. Nishino and J. Spain, Nat. Prod. Rep., 2011, 28, 152 RSC.
- For a recent example: A. Raja, B.-C. Hong, J.-H. Liao and G.-H. Lee, Org. Lett., 2016, 18, 1760 CrossRef CAS PubMed.
- For a recent review of asymmetric organocatalysis on nitro compounds, see: L. Arezki, N. R. Arezki, A. Dell'Isola and A. J. A. Cobb, Synthesis, 2013, 45, 2627 CrossRef.
- For the related asymmetric formal [2 + 2 + 2] annulations:
(a) D. Shi, Y. Xie, H. Zhou, C. Xia and H. Huang, Angew. Chem., Int. Ed., 2012, 51, 1248 CrossRef CAS PubMed;
(b) S. Varga, G. Jakab, L. Drahos, T. Holczbauer, M. Czugler and T. Soós, Org. Lett., 2011, 13, 5416 CrossRef CAS PubMed;
(c) W. Raimondi, A. S. Dugue, S. Goudedranche, A. Quintard, T. Constantieux, X. Bugaut, D. Bonne and J. Rodriguez, Synthesis, 2013, 45, 1659 CrossRef CAS;
(d) A. Nakamura, S. Lectard, D. Hashizume, Y. Hamashima and M. Sodeoka, J. Am. Chem. Soc., 2010, 132, 4036 CrossRef CAS PubMed;
(e) Z. Mao, Y. Jia, Z. Xu and R. Wang, Adv. Synth. Catal., 2012, 354, 1401 CrossRef CAS.
- 2-Phenyl-1,3-indandione was the only cyclopentane-1,3-dione derivative reported for the reaction with nitroalkene, see: A. S. Smirnov, S. V. Makarenko, V. M. Berestovitskaya, A. I. Pekki and K. S. Konovalenko, Russ. J. Org. Chem., 2006, 42, 1242 CrossRef CAS.
-
(a) C.-H. Lin, B.-C. Hong and G.-H. Lee, RSC Adv., 2016, 6, 8243 RSC;
(b) V.-W. Yang, B.-C. Hong, H.-K. Kao, T.-H. Tu, J.-Y. Shen, C.-L. Chen, G.-H. Lee and P.-T. Chou, Org. Lett., 2015, 17, 5816 CrossRef CAS PubMed;
(c) A. Raja, B.-C. Hong and G.-H. Lee, Org. Lett., 2014, 16, 5756 CrossRef CAS PubMed;
(d) N. S. Dange, B.-C. Hong and G.-H. Lee, RSC Adv., 2014, 4, 59706 RSC;
(e) D.-H. Jhuo, B.-C. Hong, C.-W. Chang and G.-H. Lee, Org. Lett., 2014, 16, 2724 CrossRef CAS PubMed;
(f) B.-C. Hong, C.-W. Lin, W.-K. Liao and G.-H. Lee, Org. Lett., 2013, 15, 6258 CrossRef CAS PubMed;
(g) N. S. Dange, B.-C. Hong, C.-C. Lee and G.-H. Lee, Org. Lett., 2013, 15, 3914 CrossRef CAS PubMed;
(h) B.-C. Hong, W.-K. Liao, N. S. Dange and J.-H. Liao, Org. Lett., 2013, 15, 468 CrossRef CAS PubMed.
- B.-C. Hong, in Constructing Molecular Complexity and Diversity by Cycloaddition Reactions of Fulvenes, Cross Conjugation: Modern Dendralene, Radialene and Fulvene Chemistry, ed. H. Hopf and N. S. Sherburn, Wiley, 2016, ch. 7, pp. 249–300 Search PubMed.
- For recent reviews:
(a) H. Pellissier, Tetrahedron, 2012, 68, 2197 CrossRef CAS;
(b) B.-C. Hong, in Enantioselective Organocatalyzed Reactions II, ed. R. Mahrwald, Springer, Dordrecht, 2011, ch. 3, pp. 187–224 Search PubMed;
(c) A. Moyano and R. Rios, Chem. Rev., 2011, 111, 4703 CrossRef CAS PubMed.
- For recent reviews, see:
(a) C. M. R. Volla, I. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390 CrossRef CAS PubMed;
(b) B.-C. Hong and N. S. Dange, in Cascade Reactions in Stereoselective Synthesis, Stereoselective Synthesis of Drugs and Natural Products, ed. V. Andrushko and N. Andrushko, Wiley, 2013, ch. 21, pp. 581–621 Search PubMed.
- For recent reviews: B.-C. Hong, A. Raja and V. M. Sheth, Synthesis, 2015, 47, 3257 CrossRef CAS.
- For recent asymmetric organocatalytic synthesis of functionalized compounds bearing five stereocenters via a one-pot or via casacde (domino) reactions, see:
(a) P. Chauhan, G. Urbanietz, G. Raabe and D. Enders, Chem. Commun., 2014, 50, 6853 RSC;
(b) P. Chauhan, S. Mahajan, G. Raabe and D. Enders, Chem. Commun., 2015, 51, 2270 RSC;
(c) L.-H. Zou, A. R. Philipps, G. Raabe and D. Enders, Chem.–Eur. J., 2015, 21, 1004 CrossRef CAS PubMed;
(d) D. Enders, A. Greb, K. Deckers, P. Selig and C. Merkens, Chem.–Eur. J., 2012, 18, 10226 CrossRef CAS PubMed;
(e) B.-C. Hong, N. S. Dange, C.-F. Ding and J.-H. Liao, Org. Lett., 2012, 14, 448 CrossRef CAS PubMed;
(f) B.-C. Hong, N. S. Dange, C.-S. Hsu, J.-H. Liao and G.-H. Lee, Org. Lett., 2011, 13, 1338 CrossRef CAS PubMed, and ref. 12b. For the examples bearing six sterocenters:
(g) P. Chauhan, S. Mahajan, G. Raabe and D. Enders, Chem. Commun., 2015, 51, 2270 RSC;
(h) L.-H. Zou, A. R. Philipps, G. Raabe and D. Enders, Chem.–Eur. J., 2015, 21, 1004 CrossRef CAS PubMed;
(i) D. Enders, A. Greb, K. Deckers, P. Seligand and C. Merkens, Chem.–Eur. J., 2012, 18, 10226 CrossRef CAS PubMed , and ref. 12b and g.
- Although 1 dissolved well in H2O, the nitrostyrene was not soluble in water; the reaction of 1 and nitrostyrene in water gave no observation of the product.
-
(a) V. A. Soloshonok and K. D. Klika, Helv. Chim. Acta, 2014, 97, 1583 CrossRef CAS;
(b) A. E. Sorochinsky and V. A. Soloshonok, Top. Curr. Chem., 2013, 341, 301 CrossRef CAS PubMed;
(c) V. A. Soloshonok, C. Roussel, O. Kitagawa and A. E. Sorochinsky, Chem. Soc. Rev., 2012, 41, 4180 RSC;
(d) J. Han, D. J. Nelson, A. E. Sorochinsky and V. A. Soloshonok, Curr. Org. Synth., 2011, 8, 310 CrossRef CAS;
(e) V. A. Soloshonok, H. Ueki, M. Yasumoto, S. Mekala, J. S. Hirschi and D. A. Singleton, J. Am. Chem. Soc., 2007, 129, 12112 CrossRef CAS PubMed;
(f) V. A. Soloshonok, Angew. Chem., Int. Ed., 2006, 45, 766 CrossRef CAS PubMed.
- The subject of SDE is particularly interesting, especially as related to prebiotic chemistry, providing a conceivable answer to a long-standing enigma which has puzzled scientists: what is the origin of the chirality of molecules in nature, see: A. Guijarro and M. Yus, The Origin of Chirality in the Molecules of Life, Royal Society of Chemistry, Cambridge, 2009 Search PubMed.
- The data were collected using CuKα (λ = 1.54178 Å) X-ray radiation. The Flack parameter was in accordance with the absolute structure assigned, absolute structure parameter = 0.06(3). Crystal system: orthorhombic, space group: P212121.
- Moreover, the above two crystals investigated by X-ray (CCDC 1488798 and CCDC 1488808) were individually subjected to HPLC analysis, comparing and co-injecting with the previous samples, with the conclusion that the chiral X-ray structures obtained herein are (−)-3i.†.
- For review in the separation of non-racemic mixtures of enantiomers using fractional crystallization, see: F. Faigl, E. Fogassy, M. Nógrádi, E. Pálovics and J. Schindler, Org. Biomol. Chem., 2010, 8, 947 CAS.
- For select examples, see:
(a) G. Springuel and T. Leyssens, Cryst. Growth Des., 2012, 12, 3374 CrossRef CAS;
(b) J. E. Hein, B. H. Cao, M. W. Meijden, M. Leeman and R. M. Kellogg, Org. Process Res. Dev., 2013, 17, 946 CrossRef CAS;
(c) Y. Wang and A. Chen, Crystallization-Based Separation Of Enantiomers, in Stereoselective Synthesis of Drugs and Natural Products, ed. V. Andrushko, and N. Andrushko, Wiley, 2013, ch. 56, pp. 1663–1682 Search PubMed;
(d) L. Pasteur, Ann. Chim. Phys., 1848, 24, 442 Search PubMed.
-
(a) H. Zhao, W. Q. Ong, F. Zhou, X. Fang, X. Chen, S. F. Y. Li, H. Su, N.-J. Chob and H. Zeng, Chem. Sci., 2012, 3, 2042 RSC;
(b) D. G. Blackmond, Cold Spring Harbor Perspect. Biol., 2010, 2, a002147 Search PubMed;
(c) O. J. Vogl, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1299 CrossRef CAS;
(d) J. Bailey, A. Chrysostomou, J. H. Hough, T. M. Gledhill, A. McCall, S. Clark, F. Ménard and M. Tamura, Science, 1998, 281, 672 CrossRef CAS PubMed;
(e) J. Podlech, Angew. Chem., Int. Ed., 1999, 38, 477 CrossRef CAS;
(f) V. A. Pavlov and E. I. Klabunovskii, Curr. Org. Chem., 2014, 18, 93 CrossRef CAS.
- For an excellent example and a review, see:
(a) R. R. E. Steendam, J. M. M. Verkade, T. J. B. van Benthem, H. Meekes, W. J. P. van Enckevort, J. Raap, P. J. T. Floris, F. P. Rutjes and E. Vlieg, Nat. Commun., 2014, 5, 5543 CrossRef CAS PubMed;
(b) I. Weissbuch and M. Lahav, Chem. Rev., 2011, 111, 3236 CrossRef CAS PubMed.
- With the internal standard of 1,3,5-trimethoxybenzene, the ratio of 3a/5a was found to remain similar along with the progress of the reaction time, i.e., the isomerization of 5a to 3a was not observed.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a,
Arun Rajaa,
Chun-Wei Changa and
Gene-Hsiang Leeb
*a,
Arun Rajaa,
Chun-Wei Changa and
Gene-Hsiang Leeb

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were fruitless and we observed only trace amounts of product 3a, Table 1, entry 12–16. The reaction conditions of DIPEA (150 mol%) at 0.2 M scale of 1 (Table 1, entry 9) were the best ones since the reactions at diluted conditions provided lower yields, (Table 1, entries 17–19). The reaction of two equivalents of 1 and one equivalent of 2a with DIPEA (80 mol%) in CH3CN for 2 h afforded 22% yield of the intermediate 4a (Table 1, entry 20). Notably, 4a was unstable and it gradually decomposed back to 1 and 2a after standing at room temperature for a few days.
2) were fruitless and we observed only trace amounts of product 3a, Table 1, entry 12–16. The reaction conditions of DIPEA (150 mol%) at 0.2 M scale of 1 (Table 1, entry 9) were the best ones since the reactions at diluted conditions provided lower yields, (Table 1, entries 17–19). The reaction of two equivalents of 1 and one equivalent of 2a with DIPEA (80 mol%) in CH3CN for 2 h afforded 22% yield of the intermediate 4a (Table 1, entry 20). Notably, 4a was unstable and it gradually decomposed back to 1 and 2a after standing at room temperature for a few days.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)c
5)c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43
43





