DOI:
10.1039/C6RA19030H
(Paper)
RSC Adv., 2016,
6, 95306-95313
Design and synthesis of the polymers based on alkylthiophenyl side chains and variant acceptor moieties for polymer solar cells†
Received
27th July 2016
, Accepted 9th September 2016
First published on 29th September 2016
Abstract
Three donor–acceptor (D–A) copolymers PSB-DFBT, PSB-DTDFBT and PSB-FTT, consisting of alkylthiophenyl group-substituted benzo[1,2-b:4,5-b′]dithiophene (SB) as the donor moieties and different electron-deficient units as the acceptor moieties, have been designed and synthesized. The effects of the variant acceptor moieties on the photophysical, electrochemical and photovoltaic properties of the polymers were investigated. Compared with the polymers PSB-DFBT and PSB-DTDFBT with 5,6-difluorobenzo[c][1,2,5]thiadiazole as the acceptor moieties, the polymer PSB-FTT with 2-ethylhexyl-3-fluorothieno[3,4-b]thiophene-2-carboxylate (FTT) as the acceptor moieties shows smaller band gap, stronger light harvesting capacity, better appropriate microphase separation with (7,7)-phenyl-C71-butyric acid methyl ester (PC71BM), smaller domain size, more effective exciton dissociation and higher hole mobility. Therefore, the PSB-FTT-based bulk heterojunction solar cell exhibits a higher power conversion efficiency of 4.45% than that of the PSB-DFBT- (1.88%) and PSB-DTDFBT-based devices (0.48%).
Introduction
Polymer solar cells (PSCs) have drawn significant attention from both the academic and industrial communities over the past decade because of their potential for low-cost, flexible, and light-weight devices.1–4 The power conversion efficiency (PCE) of PSC devices has been gradually improved due to the exploitation of novel photovoltaic materials,5–18 the application of new interface materials,19–23 and the optimization of device structure.24–26 Recently, the PCE value of single-junction PSCs is up to 11.7%.27,28 To achieve a high photovoltaic performance, the photovoltaic material is the key, so the push–pull conjugated polymers, or the so-called donor–acceptor (D–A) alternating conjugated polymers, have been widely exploited due to its intrinsic features,29–32 such as a low band gap for fully utilizing solar energy as possible, an appropriate highest occupied molecular orbital (HOMO) energy level and lowest unoccupied molecular orbital (LUMO) energy level for realizing efficient charge separation with low energy loss, a high hole mobility for facilitating charge transport.
In the last ten years, the polymers based on benzo[1,2-b:4,5-b′]dithiophene (BDT) as D moieties have been proved to be the most important polymeric photovoltaic materials.33–36 Usually, the BDT-based polymers possess high HOMO energy levels, which lead to a low open-circuit voltage (Voc).33 For example, the most famous PTB7 only shows a HOMO energy level of −5.15 eV, which results in a low Voc value of 0.74 V.37 To depress the HOMO energy levels of the BDT-based polymers, the alkoxy groups on BDT unit were substituted by 2-alkylthienyl, p-alkylphenyl, and alkyl groups, the polymers PBDTT-DPP,38 PBDTP-DPP38 and PBnDT-FTAZ39 showed lower HOMO levels of −5.30 eV, −5.35 eV and −5.36 eV, respectively. Subsequently, Li group replaced alkylthienyl with alkylthiothienyl group as side chains to obtain the polymer PBDTT-S-TT, its HOMO energy level was reduced to −5.41 eV, and the corresponding PSC device showed a high Voc value of 0.84 V.6 Recently, Bo and his coworker reported the polymer based BDT derivative with 2-ethylhexylthiophenyl side chain as the D moieties and alkoxy benzo[c][1,2,5]thiadiazole as the A moieties, and the polymer showed a low-lying HOMO energy level of −5.41 eV.30
Herein, three D–A copolymers based on BDT derivative with 2-hexyldecylthiophenyl side chain as the D moieties and different electron-deficient units as the A moieties have been designed and synthesized. Firstly, 5,6-difluorobenzo[c][1,2,5]thiadiazole (DFBT) was introduced as the A moiety to obtain the polymer PSB-DFBT (Fig. 1). Theoretically, DFBT should possess a stronger electron-deficient property than benzo[c][1,2,5]thiadiazole and alkoxy-substituted benzo[c][1,2,5]thiadiazole, so the polymer PSB-DFBT should possesses more redshifted absorption than the reported polymer P1.30 Subsequently, two 3-hexylthiophene units were inserted between D moiety and A moiety in order to improve the solubility, and the polymer PSB-DTDFBT was obtained. Finally, 2-ethylhexyl-3-fluorothieno[3,4-b]thiophene-2-carboxylate was used as the A moiety to obtain the polymer PSB-FTT. Furthermore, the effects of the variant acceptor moieties on the light-harvesting capacity, band gaps, aggregation, the morphology and hole mobility of the blend film with PC71BM, and photovoltaic performance of the PSC devices have been fully investigated.
 |
| | Fig. 1 Chemical structure of the polymers. | |
Experimental section
Materials
Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione, 4,7-dibromo-5,6-difluorobenzo[c][1,2,5]thiadiazole (M2) and 2-ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate (M4) were purchased from Derthon Optoelectronic Materials Science & Technology Co., LTD. 1-Bromo-2-hexyldecane40 and 4,7-(bis(5-bromo-4-hexyl)thiophene-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole (M3) were synthesized according to already published procedures.41 Tetrahydrofuran (THF) and toluene were refluxed over sodium and benzophenone, and distilled prior to use. DMF was dried by molecular sieve, and then distilled under reduced pressure. All the other chemicals were purchased from the commercial suppliers (Aldrich, Energy Chemical, Alfa, etc.) and used as received unless noted otherwise. Column chromatography was carried out on silica gel (Qingdao Banke Separation Materials Co., LTD., 200–300 mesh).
Characterization
1H NMR and 13C NMR spectra were recorded on a Bruker AVANCE 400 spectrometer. Molecular mass was determined by matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using a Bruker Aupoflex-III mass spectrometer. The elemental analysis result was characterized by Elementar VarioEL CHNS. UV-visible-near infrared absorption spectra (UV-vis-NIR) spectra of the polymers were recorded on a Perkin-Elmer Lambda 25 spectrometer. Thermogravimetric analyses (TGA) were performed by using a Netzsch TG 209 analyzer under nitrogen atmosphere at a heating rate of 20 °C min−1. Differential scanning calorimetric measurement (DSC) was performed on a TA DSC Q10 instrument with a heating rate of 20 °C min−1. The average molecular weight and polydispersity index (PDI) of the polymers were determined by Waters 1515 gel permeation chromatography (GPC) analysis with THF as the eluent and polystyrene as the standard. Cyclic voltammetry (CV) was conducted on an electrochemistry workstation (ZAHNER ZENNIUM) with the polymer thin film on ITO (Indium Tin Oxide) glass as the working electrode, Pt wire as the counter electrode, and Ag/AgNO3 electrode as the reference electrode in a 0.1 M tetra-n-butylammonium hexafluorophosphate acetonitrile solution at a scan rate of 100 mV s−1. Atomic force microscopy (AFM) measurements were carried out on a Digital Instruments Enviro Scope instrument operated in a tapping mode.
Fabrication and characterization of PSCs
The photovoltaic cells were constructed in a conventional sandwich structure of ITO/PEDOT:PSS (30 nm)/polymer:PC71BM (110 nm)/LiF (0.4 nm)/Al (100 nm). The patterned ITO-coated glass substrates were pre-cleaned with sequential ultrasonic agitation in detergent, deionized water, acetone, alcohol, and isopropyl alcohol for 20 min each, followed by a UVO treatment for 10 min. A thin layer (30 nm) of PEDOT:PSS was spin-coated onto the ITO glass at a speed of 3000 rpm for 30 s, and baked at 150 °C for 10 min. A mixture of the polymers and PC71BM was dissolved in o-dichlorobenzene (the polymer concentration is 15 mg mL−1) with or without 3% DIO and heated at 100 °C for 5 hours. The photosensitive layer was spin-coated on the PEDOT:PSS layer, and dried at room temperature for 2 hours in a nitrogen-filled glove box without thermal annealing treatment. The cathode of the device, consisting of 0.4 nm of LiF and 100 nm of aluminum, was thermally deposited on the top of the blend film at 5 × 10−4 Pa. The thickness of the active layer was measured by an Ambios Technology XP-100 surface profilometer. Current density–voltage (J–V) characteristics were measured by a computer controlled Keithley 2602 source meter in the dark and under AM 1.5G illumination conditions, 100 mW cm−2. The measurement of monochromatic incident photon-to-current conversion efficiency (IPCE) was performed by using a Zolix DCS300PA Data acquisition system. All these measurements were performed under ambient atmosphere at room temperature.
Results and discussion
Synthesis and characterization
The synthetic routes for the monomers and the polymers are shown in Scheme 1 and the detailed synthetic processes are presented in ESI.† The monomers M2 and M4 were used as received. The monomer M1 was synthesized by nucleophilic aromatic substitution. The structure of the monomer M1 was confirmed by 1H NMR, 13C NMR, and elemental analysis. The polymerization reaction was proceeded by the Stille coupling polymerization, and the final products PSB-DFBT, PSB-DTDFBT and PSB-FTT all exhibited excellent solubility in common organic solvents such as chloroform, THF, chlorobenzene and o-dichlorobenzene. The polymerization results and thermal properties of the polymers are summarized in Table 1. The number-average molecular weight 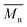 and polydispersity index (PDI) of the polymers, are
and polydispersity index (PDI) of the polymers, are 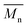 = 30.2 kDa with PDI = 1.93 for PSB-DFBT,
= 30.2 kDa with PDI = 1.93 for PSB-DFBT,  = 40.2 kDa with PDI = 2.08 for PSB-DTDFBT and
= 40.2 kDa with PDI = 2.08 for PSB-DTDFBT and  = 99.9 kDa with PDI = 2.48 for PSB-FTT, respectively. It is noticed that the molecular weight of PSB-FTT is obviously higher those of the polymers based on 5,6-difluorobenzo[c][1,2,5]thiadiazole under the same polymerization condition, which should be attributed to its better solubility derived from 2-ethylhexyl-3-fluorothieno[3,4-b]thiophene-2-carboxylate. Since PSB-FTT possesses obviously higher molecular weight than the other polymers, it would be easy to achieve a higher hole mobility and a larger short circuit current densities (Jsc).42
= 99.9 kDa with PDI = 2.48 for PSB-FTT, respectively. It is noticed that the molecular weight of PSB-FTT is obviously higher those of the polymers based on 5,6-difluorobenzo[c][1,2,5]thiadiazole under the same polymerization condition, which should be attributed to its better solubility derived from 2-ethylhexyl-3-fluorothieno[3,4-b]thiophene-2-carboxylate. Since PSB-FTT possesses obviously higher molecular weight than the other polymers, it would be easy to achieve a higher hole mobility and a larger short circuit current densities (Jsc).42
 |
| | Scheme 1 Synthetic route of the polymers. | |
Table 1 Polymerization results and thermal properties of the polymers
| Polymer |
Yields (%) |

(kDa) |
PDI |
Td (°C) |
| PSB-DFBT |
78.2 |
30.2 |
1.93 |
352 |
| PSB-DTDFBT |
76.8 |
40.2 |
2.08 |
344 |
| PSB-FTT |
81.6 |
99.9 |
2.48 |
356 |
Thermal properties
The thermal properties of the polymers were determined by thermogravimetric analysis (TGA). As shown in Fig. S12† and Table 1, the degradation temperatures (Td) of the polymers PSB-DFBT, PSB-DTDFBT and PSB-FTT with 5% weight loss are 352, 344 and 356 °C, respectively. Obviously, the thermal stability of these polymers is good enough for the application in photovoltaic devices.
Optical properties
The optical properties of the polymers were investigated by UV-vis-NIR spectra and photoluminescence (PL) spectroscopy. Fig. 2 shows the absorption spectra of PSB-DFBT, PSB-DTDFBT and PSB-FTT in diluted chloroform solution and thin film, and the correlative data are summarized in Table 2. As shown in Fig. 2a, the absorption spectra of PSB-DFBT, PSB-DTDFBT and PSB-FTT in diluted chloroform solution show two distinct absorption bands. The first absorption band at shorter wavelength region (300–500 nm), with the absorption peak wavelength at 318 nm for PSB-DFBT, 329 nm for PSB-DTDFBT, 310 nm for PSB-FTT can be identified with the π–π* transition of the polymer. The another absorption band at longer wavelength region (500–800 nm), with the maximum absorption peak (λs,max) at 573 nm for PSB-DFBT, 538 nm for PSB-DTDFBT, and 608 nm for PSB-FTT, respectively, is attributed to intramolecular charge transfer (ICT) between the electron-donating units and the electron-withdrawing units.43 The corresponding maximum absorption coefficients are 4.0 × 104, 3.1 × 104, and 7.0 × 104 mL g−1 cm−1 for PSB-DFBT, PSB-DTDFBT, and PSB-FTT, respectively. Synchronously, a weak shoulder peak between 620 and 700 nm can be observed from Fig. 2a, which is attributed to the intermolecular charge transfer. It indicates that every polymer forms an intermolecular π–π stacking in solution. It is noted that the extinction coefficients of PSB-FTT in short wavelength region (300–500 nm) and long wavelength region (500–800 nm) are obviously higher than those of PSB-DFBT and PSB-DTDFBT, which indicates PSB-FTT possesses stronger absorption capacity in solution.
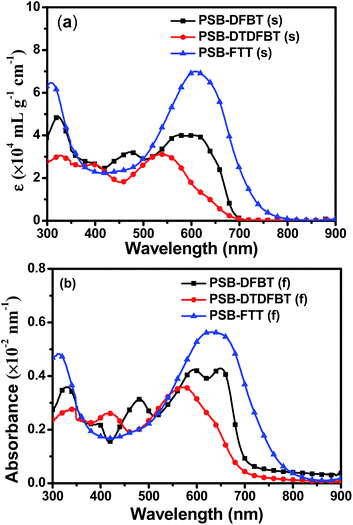 |
| | Fig. 2 UV-vis-NIR absorption spectra of the polymers in chloroform solution (a) and thin films (b). | |
Table 2 Optical and electrochemical properties of the polymers
| Polymers |
Solution |
Film |
HOMO (eV) |
LUMO (eV) |
Eecg (eV) |
| λs,max (nm) |
λf,max (nm) |
| PSB-DFBT |
573 |
595 |
−5.39 |
−3.54 |
1.84 |
| PSB-DTDFBT |
538 |
575 |
−5.30 |
−3.55 |
1.74 |
| PSB-FTT |
608 |
630 |
−5.21 |
−3.41 |
1.81 |
Compared with the absorption spectra of the polymers in solution, the absorption spectra of the films are broadened and the maximum absorption peaks (λf,max) are red-shifted (Fig. 2b). Compared with PSB-FTT, the λf,max values of PSB-DFBT and PSB-DTDFBT are blue-shifted from 630 nm to 595 nm and 575 nm respectively. It is also noted that the absorbance (defined as the absorption per unit thickness44,45) of PSB-FTT film in short wavelength region (300–350 nm) and long wavelength region (550–800 nm) is obviously higher than those of PSB-DFBT and PSB-DTDFBT films, which indicates PSB-FTT possesses stronger light harvest capacity. It implies that the polymer solar cell based on PSB-FTT could possess higher Jsc and PCE value. According to the optical absorption band edge of PSB-DFBT (719 nm), PSB-DTDFBT (709 nm) and PSB-FTT (782 nm), calculated from the absorption spectra of the films (Fig. 2b), the optical band gaps (Eoptg) of PSB-DFBT, PSB-DTDFBT and PSB-FTT can be obtained to be 1.72, 1.74 and 1.59 eV, respectively.
To investigate the exciton dissociation of the polymers, the photoluminescence (PL) spectra of PSB-DFBT, PSB-DTDFBT and PSB-FTT films, and their corresponding composite thin films with PC71BM were performed and shown in (Fig. 3). The excitation wavelengths are the maximum absorption peaks of the polymer films, which are 595 nm for PSB-DFBT, 575 nm for PSB-DTDFBT and 630 nm for PSB-FTT, respectively. As shown in Fig. 3, three pure polymer films all show strong PL emission. Compared with the pristine film, the blend film of these polymer show a weaker PL emission intensity, which indicates there is a charge transfer from the polymers to PC71BM. At the same time, the difference of the PL intensity between the blend film and the corresponding polymer is obvious. The emission peak of PSB-FTT blend film almost disappears, but those of PSB-DFBT and PSB-DTDFBT blend films are still clearly visible, indicating more effective charge transfer from PSB-FTT to PC71BM and more inferior charge transfer from the other polymers to PC71BM.
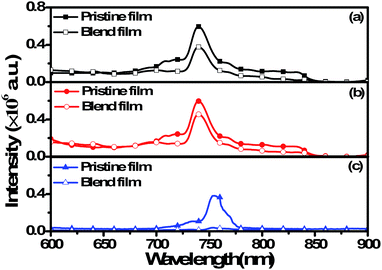 |
| | Fig. 3 Photoluminescence spectra of the polymer films and the blend films with PC71BM. (a) PSB-DFBT, (b) PSB-DTDFBT, (c) PSB-FTT. | |
Electrochemical properties
The HOMO and LUMO energy levels can be estimated from the onset oxidation potential (Eox) and the onset reduction potential (Ered) of the cyclic voltammogram (CV) respectively. The CV curves were recorded using ITO glass as the working electrode (effective area = 3 cm2), an Ag/AgNO3 electrode as the reference and Pt wire as the counter electrode. Fig. 4 shows the CV curves of the polymers and ferrocene. It can be seen that the redox potential of the ferrocene/ferrocenium couple is 0.14 V, corresponding to the absolute energy level under vacuum (−4.80 eV). Thus, the HOMO and LUMO energy levels as well as the electrochemical energy gaps (Eecg) of the polymers were calculated according to the following equations:46
| EHOMO = −e(Eox + 4.66) (eV) |
| ELUMO = −e(Ered + 4.66) (eV) |
| Eecg = e(Eox − Ered) (eV) |
 |
| | Fig. 4 Cyclic voltammograms of the polymer films. | |
The onset potentials for oxidation (Eox) were observed to be 0.73, 0.64, and 0.55 V for PSB-DFBT, PSB-DTDFBT, and PSB-FTT, respectively, corresponding the HOMO energy levels of −5.39 for PSB-DFBT, −5.30 for PSB-DTDFBT, and −5.21 eV for PSB-FTT, which are lower than the threshold HOMO level for air stable conjugated polymers (−5.2 eV). Therefore, the three polymers should be enough stable in ambient conditions. The Ereds of PSB-DFBT, PSB-DTDFBT, and PSB-FTT were found to be −1.12, −1.11 and −1.25 V, respectively. Therefore, the LUMO energy levels of them are −3.54, −3.55 and −3.41 eV. Obviously, all the polymers show higher LUMO levels than the LUMO level of the PC71BM acceptor (−4.2 eV), which imply an effective charge transfer could occur from the polymers to PC71BM.47 According to the HOMO levels and the LUMO levels, the electrochemical band gaps (Eecg) of the polymers are calculated to be 1.84 eV for PSB-DFBT, 1.74 eV for PSB-DTDFBT, and 1.81 eV for PSB-FTT.
X-ray diffraction
To investigate the effect of the chemical structure on the molecular aggregation of the polymers, wide angle X-ray diffraction (WAXRD) of the polymeric thin films was performed (Fig. 5) with Cu Kα radiation. As shown in Fig. 5, every polymer shows an indistinct 100 diffraction peaks at 3.97° for PSB-DFBT, 3.85° for PSB-DTDFBT, 3.92° for PSB-FTT, corresponding to the lamellar distance of 22.24 Å, 22.93 Å, and 22.52 Å, which indicates the polymers possess a weak lamellar stacking. Simultaneously, all the polymers exhibit a distinct diffraction peak in the broad angle region of 15° between 30°. The 010 diffraction peaks are observed at 24.39° for PSB-DFBT, 21.45° for PSB-DTDFBT, and 22.27° for PSB-FTT, corresponding to the π–π stacking distances of 3.65 Å, 4.14 Å, and 3.99 Å, respectively. It is noticed that PSB-FTT shows the strongest 010 diffraction, which indicates PSB-FTT possesses the maximum π–π stacking capability since the thickness of the polymer films is similar. In other words, the polymer PSB-FTT could be easier to obtain high phase purity in the polymer:PCBM blend film, which would be beneficial to less exciton recombination and higher hole mobility.48
 |
| | Fig. 5 WAXRD patterns of the polymers films. | |
Morphology
The morphologies of the polymers:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w
1.5, w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) blend films were investigated by AFM since the morphology of photoactive layer is crucial to obtain a high performance PSC device. As shown in Fig. 6, the PSB-DFBT and PSB-DTDFBT blend film forms serious aggregation and inhomogeneous phase separation with a RMS (surface root mean square roughness) value of 3.08 nm and 5.08 nm, respectively. However, the PSB-FTT blend film forms relatively uniform morphology and small domain size with a RMS of 1.69 nm. Moreover, the PSB-FTT blend film presents a nanoscale fibril conformation. This kind of fibrous type microphase separation is beneficial to more effective charge separation and higher charge transport compared with the other polymer blend film. Therefore, the PSB-FTT-based PSC device would possesses an higher Jsc as well as PCE values.
w) blend films were investigated by AFM since the morphology of photoactive layer is crucial to obtain a high performance PSC device. As shown in Fig. 6, the PSB-DFBT and PSB-DTDFBT blend film forms serious aggregation and inhomogeneous phase separation with a RMS (surface root mean square roughness) value of 3.08 nm and 5.08 nm, respectively. However, the PSB-FTT blend film forms relatively uniform morphology and small domain size with a RMS of 1.69 nm. Moreover, the PSB-FTT blend film presents a nanoscale fibril conformation. This kind of fibrous type microphase separation is beneficial to more effective charge separation and higher charge transport compared with the other polymer blend film. Therefore, the PSB-FTT-based PSC device would possesses an higher Jsc as well as PCE values.
 |
| | Fig. 6 AFM height images and phase images for the polymers:PC71BM blend films. (a) and (b): PSB-DFBT, (c) and (d): PSB-DTDFBT, (e) and (f): PSB-FTT. | |
Hole mobility
The hole transport properties of the blend film has a strong influence on the performance of PSCs. To investigate the hole mobility of the polymers/PC71BM blend films, the hole-only devices were fabricated with the configuration of ITO/PEDOT:PSS (30 nm)/polymer:PC71BM/MoO3 (16 nm)/Al (100 nm), and the hole mobilities of the polymer blend films were measured by using the space charge limit current (SCLC) method and summarized in Table 3. As we expected, the average hole mobility of the PSB-FTT blend film (2.0 × 10−4 cm2 V−1 s−1) is higher than that of the PSB-DFBT blend film (6.6 × 10−5 cm2 V−1 s−1) and PSB-DTDFBT blend film (4.2 × 10−5 cm2 V−1 s−1) since the former possesses more uniform morphology, nanoscale fibril conformation, smaller domain size and smaller RMS value.
Table 3 Photovoltaic properties of the PSC devices and the mobilities of the blend films (polymer: PC71BM = 1 : 1.5, w/w)
| Polymer |
Jsc mA cm−2 |
Voc, V |
FF |
PCEmax (PCEave)% |
μh,ave, cm2 V−1 s−1 |
| PSB-DFBT |
6.39 |
0.76 |
0.39 |
1.88 (1.80) |
6.6 × 10−5 |
| PSB-DTDFBT |
2.97 |
0.58 |
0.28 |
0.48 (0.41) |
4.2 × 10−5 |
| PSB-FTT |
9.50 |
0.82 |
0.57 |
4.45 (4.39) |
2.0 × 10−4 |
Photovoltaic properties
The bulk heterojunction PSCs were fabricated with the conventional sandwich structure of ITO/PEDOT:PSS (30 nm)/polymer:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5)/LiF (0.4 nm)/Al (100 nm). The active layers were spin-coated from o-dichlorobenzene solution, and the preliminary optimized results are listed in Table S1.† The current density–voltage (J–V) curves of the PSCs are shown in Fig. 7a, and the corresponding photovoltaic parameters are summarized in Table 3. As shown in Fig. 7a, the PSC devices show the Voc values of 0.76 V for PSB-DFBT, 0.58 V for PSB-DTDFBT, and 0.82 V for PSB-FTT. Though PSB-DTDFBT and PSB-DTDFBT possess lower HOMO energy levels than that of PSB-FTT, the PSC devices of the former two polymer only exhibit smaller Voc values. The probable reason could be that the serious aggregation and inhomogeneous phase separation lead to increased interface trap states and charge carrier recombination.49 As mentioned before, PSB-FTT shows the largest light harvesting capacity, the strongest π–π stacking interaction, the most effective electron transport ability to PC71BM, the most appropriate microphase separation with PC71BM, the highest hole mobility and the largest molecular weight, so the PSB-FTT-based PSC device exhibits the highest Jsc value of 9.50 mA cm−2. However, PSB-DTDFBT possesses the lowest absorbance in the film, the weakest π–π stacking interaction, the most inferior electron transport ability to PC71BM and the lowest hole mobility (4.2 × 10−5 cm2 V−1 s−1), so the PSB-DTDFBT-based PSC device only shows the lowest Jsc of 2.97 mA cm−2. The incident photon-to-current conversion efficiencies (IPCE) curves give the similar results (Fig. 7b). The PSC devices based on PSB-DFBT and PSB-DTDFBT show an IPCE response between 300 and 700 nm, and the devices show the maximum IPCE value 37.3% at 485 nm, 18.7% at 480 nm, respectively. However, the PSB-FTT-based PSC device shows broader IPCE response between 300 and 800 nm with the maximum IPCE value 54.7% at 490 nm. The integrated Jsc calculated from the IPCE results, are 6.12 mA cm−2 for PSB-DFBT, 2.83 mA cm−2 for PSB-DTDFBT, and 9.10 mA cm−2 for PSB-FTT, which are nearly in accord with the measured Jsc values within experimental error.
1.5)/LiF (0.4 nm)/Al (100 nm). The active layers were spin-coated from o-dichlorobenzene solution, and the preliminary optimized results are listed in Table S1.† The current density–voltage (J–V) curves of the PSCs are shown in Fig. 7a, and the corresponding photovoltaic parameters are summarized in Table 3. As shown in Fig. 7a, the PSC devices show the Voc values of 0.76 V for PSB-DFBT, 0.58 V for PSB-DTDFBT, and 0.82 V for PSB-FTT. Though PSB-DTDFBT and PSB-DTDFBT possess lower HOMO energy levels than that of PSB-FTT, the PSC devices of the former two polymer only exhibit smaller Voc values. The probable reason could be that the serious aggregation and inhomogeneous phase separation lead to increased interface trap states and charge carrier recombination.49 As mentioned before, PSB-FTT shows the largest light harvesting capacity, the strongest π–π stacking interaction, the most effective electron transport ability to PC71BM, the most appropriate microphase separation with PC71BM, the highest hole mobility and the largest molecular weight, so the PSB-FTT-based PSC device exhibits the highest Jsc value of 9.50 mA cm−2. However, PSB-DTDFBT possesses the lowest absorbance in the film, the weakest π–π stacking interaction, the most inferior electron transport ability to PC71BM and the lowest hole mobility (4.2 × 10−5 cm2 V−1 s−1), so the PSB-DTDFBT-based PSC device only shows the lowest Jsc of 2.97 mA cm−2. The incident photon-to-current conversion efficiencies (IPCE) curves give the similar results (Fig. 7b). The PSC devices based on PSB-DFBT and PSB-DTDFBT show an IPCE response between 300 and 700 nm, and the devices show the maximum IPCE value 37.3% at 485 nm, 18.7% at 480 nm, respectively. However, the PSB-FTT-based PSC device shows broader IPCE response between 300 and 800 nm with the maximum IPCE value 54.7% at 490 nm. The integrated Jsc calculated from the IPCE results, are 6.12 mA cm−2 for PSB-DFBT, 2.83 mA cm−2 for PSB-DTDFBT, and 9.10 mA cm−2 for PSB-FTT, which are nearly in accord with the measured Jsc values within experimental error.
 |
| | Fig. 7 J–V curves (a) and IPCE curves (b) of the PSC devices based on PSB-DFBT, PSB-DTDFBT and PSB-FTT. | |
As mentioned before, the hole mobility of the PSB-DTDFBT blend film is only 4.2 × 10−5 cm2 V−1 s−1, which would certainly results in a serious imbalance between hole mobility and electron mobility in the blend film,50 so its PSC device only exhibits the lowest FF value of 0.28. On the contrary, the PSB-FTT blend film possesses the highest hole mobility (2.0 × 10−4 cm2 V−1 s−1), indicating a relatively balanced hole mobility and electron mobility in the film,50 which leads to the highest FF values of 0.57. As a result, the PSC devices exhibit the PCE values of 1.88% for PSB-DFBT, 0.48% for PSB-DTDFBT, and 4.45% for PSB-FTT.
Conclusion
In summary, three conjugated polymers based on alkylthiophenyl group-substituted benzo[1,2-b:4,5-b′]dithiophene as the D moieties have been designed and synthesized, and the effects of the variant acceptor moieties on the photovoltaic performance of the PSC devices have been fully investigated. The results indicate that the polymer PSB-FTT with 2-ethylhexyl-3-fluorothieno[3,4-b]thiophene-2-carboxylate as the A moieties shows larger light harvesting capacity, stronger π–π stacking interaction, more effective electron transport ability to PC71BM, more appropriate microphase separation with PC71BM, higher hole mobility and larger molecular weight than the polymers based on 5,6-difluorobenzo[c][1,2,5]thiadiazole as the A moieties, so the PSB-FTT-based PSC device shows higher Jsc and PCE values. This work not only reports a series of new polymers based on alkylthiophenyl side chains, but also brings an insight how the acceptor structure affects the photovoltaic performance of the corresponding PSCs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51573153), the Natural Science Foundation of Hunan Province of China (2015JJ2141), the Scientific Research Fund of Hunan Provincial Education Department (15A180) and Hunan Provincial Innovation Foundation for Postgraduate (CX2015B216).
References
- H. Bin, Z.-G. Zhang, L. Gao, S. Chen, L. Zhong, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 4657–4664 CrossRef CAS PubMed.
- L. Bian, E. Zhu, J. Tang, W. Tang and F. Zhang, Prog. Polym. Sci., 2012, 37, 1292–1331 CrossRef CAS.
- Z.-G. Zhang and Y. Li, Sci. China: Chem., 2015, 58, 192–209 CrossRef CAS.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef CAS PubMed.
- S. Li, T. Yuan, G. Tu, J. Zhang and Z. Li, Polym. Chem., 2015, 6, 7436–7446 RSC.
- C. Cui, W.-Y. Wong and Y. Li, Energy Environ. Sci., 2014, 7, 2276–2284 CAS.
- M. Zhang, X. Guo, S. Zhang and J. Hou, Adv. Mater., 2014, 26, 1118–1123 CrossRef CAS PubMed.
- J. Yuan, Y. Zou, R. Cui, Y.-H. Chao, Z. Wang, M. Ma, Y. He, Y. Li, A. Rindgen, W. Ma, D. Xiao, Z. Bo, X. Xu, L. Li and C.-S. Hsu, Macromolecules, 2015, 48, 4347–4356 CrossRef CAS.
- L. Huo, T. Liu, B. Fan, Z. Zhao, X. Sun, D. Wei, M. Yu, Y. Liu and Y. Sun, Adv. Mater., 2015, 27, 6969–6975 CrossRef CAS PubMed.
- G. Zhang, J. Zhang, G. Ding, J. Guo, H. Lu, L. Qiu and W. Ma, Polymer, 2016, 93, 213–220 CrossRef CAS.
- Y. Li, K. Yao, H.-L. Yip, F.-Z. Ding, Y.-X. Xu, X. Li, Y. Chen and A. K. Y. Jen, Adv. Funct. Mater., 2014, 24, 3631–3638 CrossRef CAS.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C.-C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed.
- H. J. Son, L. Lu, W. Chen, T. Xu, T. Zheng, B. Carsten, J. Strzalka, S. B. Darling, L. X. Chen and L. Yu, Adv. Mater., 2013, 25, 838–843 CrossRef CAS PubMed.
- W. Yue, R. S. Ashraf, C. B. Nielsen, E. Collado-Fregoso, M. R. Niazi, S. A. Yousaf, M. Kirkus, H.-Y. Chen, A. Amassian, J. R. Durrant and I. McCulloch, Adv. Mater., 2015, 27, 4702–4707 CrossRef CAS PubMed.
- L. Huo, T. Liu, X. Sun, Y. Cai, A. J. Heeger and Y. Sun, Adv. Mater., 2015, 27, 2938–2944 CrossRef CAS PubMed.
- C. Wang, B. Zhao, Z. Cao, P. Shen, Z. Tan, X. Li and S. Tan, Chem. Commun., 2013, 49, 3857–3859 RSC.
- Y.-X. Xu, C.-C. Chueh, H.-L. Yip, F.-Z. Ding, Y.-X. Li, C.-Z. Li, X. Li, W.-C. Chen and A. K. Y. Jen, Adv. Mater., 2012, 24, 6356–6361 CrossRef CAS PubMed.
- T. L. Nguyen, H. Choi, S. J. Ko, M. A. Uddin, B. Walker, S. Yum, J. E. Jeong, M. H. Yun, T. J. Shin, S. Hwang, J. Y. Kim and H. Y. Woo, Energy Environ. Sci., 2014, 7, 3040–3051 CAS.
- K. Zhang, Z. Hu, R. Xu, X.-F. Jiang, H.-L. Yip, F. Huang and Y. Cao, Adv. Mater., 2015, 27, 3607–3613 CrossRef CAS PubMed.
- C. Sun, Z. Wu, H.-L. Yip, H. Zhang, X.-F. Jiang, Q. Xue, Z. Hu, Z. Hu, Y. Shen, M. Wang, F. Huang and Y. Cao, Adv. Energy Mater., 2016, 6, 1501534 CrossRef.
- D. Yang, L. Zhou, W. Yu, J. Zhang and C. Li, Adv. Energy Mater., 2014, 4, 1400591 CrossRef.
- C.-Z. Li, C.-C. Chueh, F. Ding, H.-L. Yip, P.-W. Liang, X. Li and A. K. Y. Jen, Adv. Mater., 2013, 25, 4425–4430 CrossRef CAS PubMed.
- Z. A. Tan, W. Zhang, Z. Zhang, D. Qian, Y. Huang, J. Hou and Y. Li, Adv. Mater., 2012, 24, 1476–1481 CrossRef CAS PubMed.
- J. Huang, J. H. Carpenter, C.-Z. Li, J.-S. Yu, H. Ade and A. K. Y. Jen, Adv. Mater., 2016, 28, 967–974 CrossRef CAS PubMed.
- X. Guo, M. Zhang, J. Tan, S. Zhang, L. Huo, W. Hu, Y. Li and J. Hou, Adv. Mater., 2012, 24, 6536–6541 CrossRef CAS PubMed.
- L. Zuo, S. Zhang, H. Li and H. Chen, Adv. Mater., 2015, 27, 6983–6989 CrossRef CAS PubMed.
- H. Zhou, Y. Zhang, C. K. Mai, S. D. Collins, G. C. Bazan, T. Q. Nguyen and A. J. Heeger, Adv. Mater., 2015, 27, 1767–1773 CrossRef CAS PubMed.
- F. Huang and Y. Cao, Acta Polym. Sin., 2016, 399–401 CAS.
- S. Zhang, L. Ye and J. Hou, Adv. Energy Mater., 2016, 60, 1502529–1502549 CrossRef.
- X. Gong, G. Li, C. Li, J. Zhang and Z. Bo, J. Mater. Chem. A, 2015, 3, 20195–20200 CAS.
- H. Liu, F. Wu, B. Zhao, L. Meng, G. Wang, J. Zhang, P. Shen and S. Tan, Dyes Pigm., 2015, 120, 44–51 CrossRef CAS.
- C. Cui, Z. He, Y. Wu, X. Cheng, H. Wu, Y. Li, Y. Cao and W.-Y. Wong, Energy Environ. Sci., 2016, 9, 885–891 CAS.
- L. Lu and L. Yu, Adv. Mater., 2014, 26, 4413–4430 CrossRef CAS PubMed.
- Y. Nie, B. Zhao, P. Tang, P. Jiang, Z. Tian, P. Shen and S. Tan, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3604–3614 CrossRef CAS.
- H.-Y. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649–653 CrossRef CAS.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 Search PubMed.
- Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135–E138 CrossRef CAS PubMed.
- L. Dou, J. Gao, E. Richard, J. You, C.-C. Chen, K. C. Cha, Y. He, G. Li and Y. Yang, J. Am. Chem. Soc., 2012, 134, 10071–10079 CrossRef CAS PubMed.
- S. C. Price, A. C. Stuart, L. Yang, H. Zhou and W. You, J. Am. Chem. Soc., 2011, 133, 4625–4631 CrossRef CAS PubMed.
- R. Hou, B. Zhao, F. Wu, G. Wang, T. Shen, H. Guo, J. Zhang, H. Chen and S. Tan, Org. Electron., 2015, 20, 142–149 CrossRef CAS.
- H. Guo, T. Shen, F. Wu, G. Wang, L. Ye, Z. Liu, B. Zhao and S. Tan, RSC Adv., 2016, 6, 13177–13184 RSC.
- C. Liu, K. Wang, X. Hu, Y. Yang, C. H. Hsu, W. Zhang, S. Xiao, X. Gong and Y. Cao, ACS Appl. Mater. Interfaces, 2013, 5, 12163–12167 CAS.
- L. Meng, F. Wu, H. Liu, B. Zhao, J. Zhang, J. Zhong, Y. Pei, H. Chen and S. Tan, RSC Adv., 2015, 5, 14540–14546 RSC.
- Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, 135–138 CrossRef PubMed.
- F. Wu, R. Hou, L. Yang, B. Zhao and S. Tan, Sci. China: Chem., 2016, 59, 466–471 CrossRef CAS.
- Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243–248 CrossRef CAS.
- J. Yuan, Y. Zou, R. Cui, Y.-H. Chao, Z. Wang, M. Ma, Y. He, Y. Li, A. Rindgen, W. Ma, D. Xiao, Z. Bo, X. Xu, L. Li and C.-S. Hsu, Macromolecules, 2015, 48, 4347–4356 CrossRef CAS.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- N. K. Elumalai and A. Uddin, Energy Environ. Sci., 2016, 9, 391–410 CAS.
- L. M. Andersson, C. Müller, B. H. Badada, F. Zhang, U. Würfel and O. Inganäs, J. Appl. Phys., 2011, 110, 024509 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic processes, TGA curves, device optimization. See DOI: 10.1039/c6ra19030h |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *ab and
Songting Tanb
*ab and
Songting Tanb
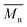 and polydispersity index (PDI) of the polymers, are
and polydispersity index (PDI) of the polymers, are 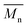 = 30.2 kDa with PDI = 1.93 for PSB-DFBT,
= 30.2 kDa with PDI = 1.93 for PSB-DFBT,  = 40.2 kDa with PDI = 2.08 for PSB-DTDFBT and
= 40.2 kDa with PDI = 2.08 for PSB-DTDFBT and  = 99.9 kDa with PDI = 2.48 for PSB-FTT, respectively. It is noticed that the molecular weight of PSB-FTT is obviously higher those of the polymers based on 5,6-difluorobenzo[c][1,2,5]thiadiazole under the same polymerization condition, which should be attributed to its better solubility derived from 2-ethylhexyl-3-fluorothieno[3,4-b]thiophene-2-carboxylate. Since PSB-FTT possesses obviously higher molecular weight than the other polymers, it would be easy to achieve a higher hole mobility and a larger short circuit current densities (Jsc).42
= 99.9 kDa with PDI = 2.48 for PSB-FTT, respectively. It is noticed that the molecular weight of PSB-FTT is obviously higher those of the polymers based on 5,6-difluorobenzo[c][1,2,5]thiadiazole under the same polymerization condition, which should be attributed to its better solubility derived from 2-ethylhexyl-3-fluorothieno[3,4-b]thiophene-2-carboxylate. Since PSB-FTT possesses obviously higher molecular weight than the other polymers, it would be easy to achieve a higher hole mobility and a larger short circuit current densities (Jsc).42

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w
1.5, w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) blend films were investigated by AFM since the morphology of photoactive layer is crucial to obtain a high performance PSC device. As shown in Fig. 6, the PSB-DFBT and PSB-DTDFBT blend film forms serious aggregation and inhomogeneous phase separation with a RMS (surface root mean square roughness) value of 3.08 nm and 5.08 nm, respectively. However, the PSB-FTT blend film forms relatively uniform morphology and small domain size with a RMS of 1.69 nm. Moreover, the PSB-FTT blend film presents a nanoscale fibril conformation. This kind of fibrous type microphase separation is beneficial to more effective charge separation and higher charge transport compared with the other polymer blend film. Therefore, the PSB-FTT-based PSC device would possesses an higher Jsc as well as PCE values.
w) blend films were investigated by AFM since the morphology of photoactive layer is crucial to obtain a high performance PSC device. As shown in Fig. 6, the PSB-DFBT and PSB-DTDFBT blend film forms serious aggregation and inhomogeneous phase separation with a RMS (surface root mean square roughness) value of 3.08 nm and 5.08 nm, respectively. However, the PSB-FTT blend film forms relatively uniform morphology and small domain size with a RMS of 1.69 nm. Moreover, the PSB-FTT blend film presents a nanoscale fibril conformation. This kind of fibrous type microphase separation is beneficial to more effective charge separation and higher charge transport compared with the other polymer blend film. Therefore, the PSB-FTT-based PSC device would possesses an higher Jsc as well as PCE values.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5)/LiF (0.4 nm)/Al (100 nm). The active layers were spin-coated from o-dichlorobenzene solution, and the preliminary optimized results are listed in Table S1.† The current density–voltage (J–V) curves of the PSCs are shown in Fig. 7a, and the corresponding photovoltaic parameters are summarized in Table 3. As shown in Fig. 7a, the PSC devices show the Voc values of 0.76 V for PSB-DFBT, 0.58 V for PSB-DTDFBT, and 0.82 V for PSB-FTT. Though PSB-DTDFBT and PSB-DTDFBT possess lower HOMO energy levels than that of PSB-FTT, the PSC devices of the former two polymer only exhibit smaller Voc values. The probable reason could be that the serious aggregation and inhomogeneous phase separation lead to increased interface trap states and charge carrier recombination.49 As mentioned before, PSB-FTT shows the largest light harvesting capacity, the strongest π–π stacking interaction, the most effective electron transport ability to PC71BM, the most appropriate microphase separation with PC71BM, the highest hole mobility and the largest molecular weight, so the PSB-FTT-based PSC device exhibits the highest Jsc value of 9.50 mA cm−2. However, PSB-DTDFBT possesses the lowest absorbance in the film, the weakest π–π stacking interaction, the most inferior electron transport ability to PC71BM and the lowest hole mobility (4.2 × 10−5 cm2 V−1 s−1), so the PSB-DTDFBT-based PSC device only shows the lowest Jsc of 2.97 mA cm−2. The incident photon-to-current conversion efficiencies (IPCE) curves give the similar results (Fig. 7b). The PSC devices based on PSB-DFBT and PSB-DTDFBT show an IPCE response between 300 and 700 nm, and the devices show the maximum IPCE value 37.3% at 485 nm, 18.7% at 480 nm, respectively. However, the PSB-FTT-based PSC device shows broader IPCE response between 300 and 800 nm with the maximum IPCE value 54.7% at 490 nm. The integrated Jsc calculated from the IPCE results, are 6.12 mA cm−2 for PSB-DFBT, 2.83 mA cm−2 for PSB-DTDFBT, and 9.10 mA cm−2 for PSB-FTT, which are nearly in accord with the measured Jsc values within experimental error.
1.5)/LiF (0.4 nm)/Al (100 nm). The active layers were spin-coated from o-dichlorobenzene solution, and the preliminary optimized results are listed in Table S1.† The current density–voltage (J–V) curves of the PSCs are shown in Fig. 7a, and the corresponding photovoltaic parameters are summarized in Table 3. As shown in Fig. 7a, the PSC devices show the Voc values of 0.76 V for PSB-DFBT, 0.58 V for PSB-DTDFBT, and 0.82 V for PSB-FTT. Though PSB-DTDFBT and PSB-DTDFBT possess lower HOMO energy levels than that of PSB-FTT, the PSC devices of the former two polymer only exhibit smaller Voc values. The probable reason could be that the serious aggregation and inhomogeneous phase separation lead to increased interface trap states and charge carrier recombination.49 As mentioned before, PSB-FTT shows the largest light harvesting capacity, the strongest π–π stacking interaction, the most effective electron transport ability to PC71BM, the most appropriate microphase separation with PC71BM, the highest hole mobility and the largest molecular weight, so the PSB-FTT-based PSC device exhibits the highest Jsc value of 9.50 mA cm−2. However, PSB-DTDFBT possesses the lowest absorbance in the film, the weakest π–π stacking interaction, the most inferior electron transport ability to PC71BM and the lowest hole mobility (4.2 × 10−5 cm2 V−1 s−1), so the PSB-DTDFBT-based PSC device only shows the lowest Jsc of 2.97 mA cm−2. The incident photon-to-current conversion efficiencies (IPCE) curves give the similar results (Fig. 7b). The PSC devices based on PSB-DFBT and PSB-DTDFBT show an IPCE response between 300 and 700 nm, and the devices show the maximum IPCE value 37.3% at 485 nm, 18.7% at 480 nm, respectively. However, the PSB-FTT-based PSC device shows broader IPCE response between 300 and 800 nm with the maximum IPCE value 54.7% at 490 nm. The integrated Jsc calculated from the IPCE results, are 6.12 mA cm−2 for PSB-DFBT, 2.83 mA cm−2 for PSB-DTDFBT, and 9.10 mA cm−2 for PSB-FTT, which are nearly in accord with the measured Jsc values within experimental error.







