Magnetic zinc oxide nanoflower-assisted ionic liquid-based nanofluid dispersive liquid–liquid microextraction for the rapid determination of acaricides in tea infusions†
Miyi Yang,
Haozhe Zeng,
Xiaoling Wu,
Xiaoling Yang,
Wenfeng Zhou,
Sanbing Zhang,
Runhua Lu,
Jing Li and
Haixiang Gao*
Department of Applied Chemistry, China Agricultural University, Yuanmingyuan West Road 2#, Haidian District, Beijing 100193, China. E-mail: hxgao@cau.edu.cn; Fax: +86 010 62835140; Tel: +86 010 62835140
First published on 17th November 2016
Abstract
A novel dispersive liquid–liquid microextraction (DLLME) method is proposed for the detection of five acaricides (clofentezine, fenpyroximate, diafenthiuron, pyridaben and spirodiclofen) in tea infusions prior to analysis by high-performance liquid chromatography (HPLC). For this method, a nanofluid prepared by dispersing magnetic zinc oxide nanoflower (FeO4@ZnO) nanoparticles in 1-octyl-2,3-dimethylimidazolium bis(trifluoromethanesulfonimide) was used as an extractant. After extraction, the nanofluid was magnetically collected by the addition of FeO4@ZnO nanoparticles without centrifugation. In DLLME, the joint use of an ionic liquid (IL)-based nanofluid and magnetic retrieval is unprecedented and makes this microextraction process rapid and effective. The main parameters affecting the extraction efficiency were optimized by an orthogonal experiment and a single-factor experiment using tea infusions. Under the optimal conditions, the recoveries of the analytes were between 83.0 and 104.4%, the limits of detection ranged from 0.44 to 1.0 μg L−1, and the intra-day precision and inter-day precision were both lower than 7.5%. The proposed method was successfully applied to the analysis of acaricides in four tea infusions, and the recoveries were between 79.2 and 93.1%, with RSDs of 0.4 to 5.8%. The developed method is promising as an environmentally friendly, convenient, and efficient sample preparation technique.
Introduction
Tea is the most widely consumed non-alcoholic beverage in East Asian countries and has become popular throughout the world for its therapeutic properties, low caffeine content and specific aroma.1 Several agrochemicals are used in tea farming to minimize the possible effects of diseases, weeds, and pests, particularly mites, leaf-eating beetles, and caterpillars.2 Clofentezine, fenpyroximate, diafenthiuron, pyridaben and spirodiclofen, all insecticide acaricides, are widely applied to crops.3 Notably, pesticide residues can transfer from prepared tea or dried tea leaves into a tea infusion during the brewing process.4 Hence, the contamination of a tea infusion with residual acaricides may pose a potential threat to the health of tea drinkers. The maximum residue limits (MRLs) set by the EU for tea are less than 0.05 mg kg−1 for clofentezine, pyridaben and spirodiclofen and less than 0.1 mg kg−1 for fenpyroximate.5 However, tea is drunk after it is steeped in water, and tea leaves are not directly consumed. Therefore, it is necessary to monitor the quality of tea infusions. In this regard, sensitive, precise, and accessible analytical techniques are imperative for measuring acaricide levels in tea infusions.Sample preparation is a vital step to obtain accurate instrumental analysis. In addition to traditional sample preparation methods such as liquid–liquid extraction (LLE)6 and SPE,7 some novel techniques have been developed for matrix extraction and to preconcentrate agricultural chemical analytes. Dispersive liquid–liquid microextraction (DLLME) was first introduced by Rezaee et al. and has been widely applied as a powerful sample preparation technique. DLLME utilizes a ternary component solvent system consisting of an aqueous sample, a dispersive solution and an extraction solvent. The extraction solvent dissolves in the dispersive solution, and the mixture is then rapidly injected into the aqueous phase. A cloudy solution consisting of tiny droplets of extraction solvent forms almost simultaneously. Due to the enormous contact area between the analytes and the extraction solvent, rapid and effective mass transfer occurs.8 DLLME is acclaimed for its simplicity, ease of miniaturization, rapid extraction and low cost. However, chlorinated solvents, such as chloroform (CF), trichloroethylene (TCE) and tetrachloromethane (CCl4), are the most commonly used extraction solvents for this technique, which might not serve the principles of green analytical chemistry.9
Metal oxide nanoparticles have played important roles in many fields, including catalysis, separation, biosensing, semiconductors, and ceramics, due to their unique surface properties and thermal stability.10–13 Metal oxides reportedly improve separation efficiency when used as the packing material for high-performance liquid chromatography (HPLC) columns.14 Zinc oxide (ZnO) nanoparticles have been reported as a useful enhanced separation material. Ayanda et al. used ZnO nanoparticles to remove triphenyltin chloride (TPT) from dockyard wastewater.15 ZnO/G-C3N4 nanoflower-coated solid-phase microextraction fibers have also been coupled with gas chromatography-mass spectrometry (GC-MS) to detect pesticide residues.16 When ZnO is modified with ferroferric oxide, the nanoparticles exhibit magnetism in addition to their original characteristics. Magnetic metal oxide nanoparticles have been applied in analytical chemistry as well. For example, Mandal et al. used ZnO/ZnxFe3xO4 mixed oxides to adsorb and degrade organic dyes in water.17 Overall, magnetic zinc oxides have promising prospects in analytical applications.
Nanofluids, composed of nanoparticles suspended in liquid, are considered a novel type of composite material.18 Nanoparticles have been shown to enhance the mass-transfer coefficients of nanofluids, and the potential advantages of these nanostructures might be due to their intrinsically high reaction cross sections, high specific surface areas and high densities of surface functionalities.19,20 Nanofluids have also been used in a spray LLE column system to improve mass transfer.21 Moreover, some magnetic microfluids have excellent extraction abilities for DNA and organic compounds.22,23
Ionic liquids (ILs) are considered environmentally friendly solvents and have recently been broadly applied in analytical chemistry.24–26 ILs have outstanding physicochemical properties, including high polarity, low volatility, high thermal stability, variable viscosity, good chromatographic behavior and good ability to extract organic compounds.27,28 Furthermore, these liquids can be tuned for targeted functionality based on different combinations of components, which can enhance their interactions with target species and cause self-aggregation.29 All these features enable ILs to serve as potentially desirable extraction media for DLLME. Extraction methods based on IL-DLLME have been successfully used to detect benzimidazolic pesticides in soils,30 benzoylurea insecticides in wastewater,31 and triazole pesticides in rat blood.32 Meanwhile, ILs have often been used in novel solution systems and serve as vital components of dispersion medium,33 which might benefit from the strong interactions that form between nanoparticles needing dispersal and ILs. IL-based ZnO nanofluid exhibits high efficiency in recovering mercury via vortex-assisted liquid–liquid microextraction, as reported by Amde et al.34 This group also used a nanofluid consisting of ZnO dispersed in 1-hexyl-3-methylimidazolium hexafluorophosphate to extract fungicides from environmental water samples.35 However, studies using magnetic metal oxide nanofluids based on ILs are rare.
Centrifugation is an essential step for the collection of extraction solvent in most DLLME methods due to the high density of extractants; however, this operation is time-consuming.36,37 Consequently, nanoscale magnetic separation methods were developed to decrease sample preparation time and simplify experimental procedures. Shi et al. proposed a novel method for DLLME in which extractant containing target analytes is collected without centrifugation using magnetic nanoparticles (MNPs).38 The innovative use of MNPs for extractant collection has proved successful for the detection of benzoylurea insecticides and UV filters.39,40 Nevertheless, to the best of our knowledge, no reports have discussed the use of MNPs combined with an IL-based nanofluid in DLLME.
In this work, magnetic zinc oxide (ZnO@Fe3O4) nanoflowers were fabricated and characterized, and a nanofluid consisting of ZnO@Fe3O4 and [OMMIM]NTF2 was prepared. Combining the advantages of MNPs and the IL-based nanofluid, we propose a novel magnetic assisted-IL-based nanofluid-dispersive liquid–liquid microextraction (MA-ILNF-DLLME) method. With the assistance of magnetic retrieval, this method was coupled to HPLC and used for trace-level analysis of five acaricides in a tea infusion. This enabled rapid, efficient and environmentally friendly analysis. The experimental parameters of this method were optimized using real samples. Satisfactory results were obtained in the analysis of four types of tea using the proposed method under the optimized conditions.
Experimental
Reagents and materials
Clofentezine, fenpyroximate, diafenthiuron, pyridaben and spirodiclofen (purities ranging from 98% to 99%) were provided by the Agricultural Environmental Protection Institution (Tianjin, China). Ethanol and acetone were purchased from Beijing Chemical Factory (Beijing, China). Analytical-grade sodium chloride (NaCl), zinc acetate dehydrate (Zn(COOH)2), iron chloride hexahydrate (FeCl3·6H2O), anhydrous sodium sulfite (Na2SO3), and ammonium hydroxide (NH4OH) were acquired from Sinopharm Chemical Reagent Company (Beijing, China). Hexamethylenetetramine (HMT) was purchased from Tianjin Damao Chemical Reagent Factory (Tianjing, China). Lithium bis(trifluoromethylsulfonyl)amide (C2F6LiNO4S2) was purchased from Zhengzhou Alfa chemical Co. Ltd. (Henan, China). HPLC-grade acetonitrile and methanol were purchased from Dikma (Beijing, China), and deionized water was purified using a Milli-Q SP Reagent Water System (Millipore, Bedford, MA, USA). ILs, including 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonimide) ([HMIM]NTF2), 1-octyl-3-methylimidazolium hexafluorophosphate ([OMIM]PF6), 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonimide) ([OMIM]NTF2), 1-hexyl-2,3-dimethylimidazolium chloride ([HMMIM]Cl) and 1-octyl-2,3-dimethylimidazolium chloride ([OMMIM]Cl), were purchased from the Center for Green Chemistry and Catalysis, LICP, CAS (Lanzhou, China).Instrumental and analytical conditions
Chromatographic analysis was performed on an Agilent 1100 HPLC system (CA, USA), which consisted of a binary high-pressure pump, a column oven, an auto-sampler and a diode array detector (DAD). Agilent software was used to control the LC system and acquire data. Compound separation was performed on a Spursil C18 column (5 μm, 4.6 × 250 mm, Dikma Ltd.) protected by a Spursil C18 guard cartridge (5 m, 2.1 × 10 mm, Dikma) at 25 °C under isocratic conditions with an acetonitrile/water solution (74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26, v/v) as the mobile phase at a flow rate of 1 mL min−1. The UV detection wavelengths were set to 270 nm for clofentezine and 230 nm for the other four acaricides.
26, v/v) as the mobile phase at a flow rate of 1 mL min−1. The UV detection wavelengths were set to 270 nm for clofentezine and 230 nm for the other four acaricides.
A Mettler-Toledo AL104 electronic balance (Shanghai, China) was used. Samples were filtered through 0.22 μm micropore membranes (Agla, USA). A Zonkia SC-3610 low-speed refrigerated centrifuge (Anhui, China) and a KQ3200DE ultrasonicator (Kunshan, China) were used for sample treatment. The surface morphologies were studied using a JEOL JSM-7001F (Jeol, Japan) scanning electron microscope (SEM). Transmission electron microscopy (TEM) images were recorded using a Tecnai G2 F20 S-TWIN microscope (FEI, USA). X-ray diffraction (XRD) measurements were performed on a D8 Focus instrument (Bruker, Germany). Fourier-transform infrared (FTIR) spectra were recorded on a Spectrum 100 spectrometer (Perkin Elmer, USA).
Experimental design and analysis were achieved using Minitab® Release 16 Statistical Software (State College, PA, USA).
Preparation of ILs
[OMMIM][NTF2] and [HMMIM][NTF2] were synthesized according to a previous method based on an anion-exchange reaction.41 Briefly, the corresponding chloride salt of the imidazolium cation ([OMMIM]Cl and [HMMIM]Cl) and the same amount of lithium bis(trifluoromethylsulfonyl)amide were dissolved in water, and the bottom IL phase was collected and washed with deionized water 5 times. The product was dried at 60 °C for 8 h in a vacuum oven before use.Sample preparation
Huoshan Huangya, Tie Guanyin, Longjing and Maojian teas were purchased from a local market (Beijing, China). A total of 5 g of the prepared tea was immersed in 150 mL of boiled water. After brewing for 3 min, the hot aqueous extract was filtered and cooled to room temperature. To evaluate the extraction performance and optimize the extraction conditions, tea infusion samples were spiked with analytes at various concentrations. The analytes were quantified through external calibration, in which a series of standard solutions was prepared and analyzed by HPLC to obtain linear calibration plots for each analyte. The concentrations of the tested solutions were determined based on the chromatographic peak areas compared to the obtained linear equations.Preparation of ZnO@Fe3O4 nanoflowers
ZnO@Fe3O4 was prepared according to a process reported by Cao et al. with minor modifications.42 Specifically, 150 mL of an aqueous solution of Zn(COOH)2 was mixed with the same volume of an aqueous solution of HMT at an equal concentration (0.1 M) in a 500 mL flask. After mixing with magnetic stirring, the mixture was heated to 90 °C for 24 h under reflux conditions. The white ZnO powder was obtained after centrifugation, washed with deionized water and ethanol, and dried. The next step consisted of modifying ZnO with Fe3O4. A total of 80 mL of an aqueous FeCl3 solution (0.3 M) and 80 mL of an aqueous sodium sulfite solution (0.1 M) were mixed together and mechanically stirred until the color of the aqueous solution changed. A total of 2.0 g of ZnO was added to the light yellow solution with stirring. Then, 120 mL of ammonia was quickly added to the above mixture, which was maintained at 90 °C for 2 h with continuous stirring. The products were washed with deionized water and ethanol under an external magnetic field. Black powder with a metallic luster was obtained after drying at 60 °C in a vacuum oven.Preparation of IL-based ZnO@Fe3O4 nanofluid
IL–ZnO@Fe3O4 nanofluid was prepared using a two-step method for oxide nanopowders with good performance.43 The synthesized ZnO@Fe3O4 powder was dispersed in IL and vortexed to obtain a homogeneous distribution. The obtained nanofluids were then sonicated for 60 min to break up possible clusters of the nanoparticles. Fig. S1† compares the status of the original IL and the ZnO@Fe3O4 nanofluid.Microextraction procedure
The extraction procedure was easily carried out, and the status at each step in the extraction procedure is shown in Fig. 1. A total of 8 mL of the tea infusion sample was placed in a test tube (Fig. 1a). Then, 150 μL of methanol containing 50 mg of the [OMIMM]NTF2–ZnO@Fe3O4 nanofluid was quickly injected into the tube via a microsyringe to form an emulsified solution (Fig. 1b). Next, 15 mg of ZnO@Fe3O4 was added to the tube. After sealing, the mixture was vortexed for 2 min at 2800 rpm (Fig. 1c). The magnetic nanofluids that contained the target analytes were successfully gathered by the magnetic nanoparticles, and the process was accelerated by shaking. The extractant and MNPs were sedimented at the bottom using a magnet (Fig. 1d). The aqueous supernatant phase was discarded. Subsequently, the acaricides extracted by the nanofluids were desorbed from the nanoparticles by sonication for 1 min with 60 μL of acetonitrile. The MNPs were easily isolated from the solution using a magnet, and 10 μL of the collected organic solvent was used for the final HPLC analysis. | ||
| Fig. 1 Steps in the META-IL-DLLME method. (a–d) visually depict the status of each sequential step during the microextraction. | ||
Results and discussion
Characterization of ZnO@Fe3O4
The FTIR spectra of the ZnO@Fe3O4 nanoparticles and the [OMMIM][NTF2]-based nanofluid were employed to study the formation of the compounds. Fig. 2 shows that a band at approximately 400–700 cm−1 was assigned to the stretching vibration of metal–O bonds, including Zn–O and Fe–O. For the nanofluids, the absorbance peaks at 2800 and 3000 cm−1 refer to the C–H stretching vibrations of the alkyl groups at the nitrogen atoms of the imidazolium ring. The absorbance feature at approximately 3200 cm−1 consists of two bands resulting from the C–H stretching mode. The peaks at approximately 1500 to 1000 cm−1 were assigned to the stretching modes of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the ILs. These results illustrate the structural features of the MNPs and the nanofluid.
O in the ILs. These results illustrate the structural features of the MNPs and the nanofluid.
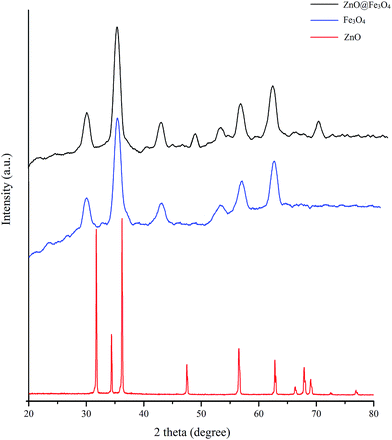
The structure of the ZnO@Fe3O4 nanoflower was further characterized by XRD (Fig. 3). To obtain a comprehensive view, the XRD patterns of pure ZnO and Fe3O4 nanoparticles were also determined. In the case of the Fe3O4 nanoparticles, distinct diffraction peaks were marked with the indices (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0), corresponding to the diffractions of Fe3O4 with a face-centered cubic crystalline phase. The diffraction peaks of the ZnO powders matched those in the hexagonal wurtzite structure, indicating the successful formation of the ZnO crystal. For the ZnO@Fe3O4 nanocomposite, the diffraction peaks observed can be clearly indexed to Fe3O4 and crystalline ZnO. The strong and sharp peaks suggest that the Fe3O4 coated on the ZnO was highly crystalline.
The morphologies of the resultant nanocomposites were investigated by SEM and TEM, and the results are shown in Fig. 4. As shown in Fig. 4A, a large quantity of ZnO nanotube bundles can be observed. As Fig. 4B indicates, the ZnO bundles were composed of closely packed nanotubes with an average diameter of approximately 1.083 μm that formed radiating structures such as flowers. Fig. 4C shows the electron backscattering diffraction pattern of ZnO@Fe3O4; an invariable phase-contrast can be observed, which is direct evidence of the uniform distribution of the ZnO-coated Fe3O4 nanoparticles. SEM images cannot reveal the fine structure of MNPs, and thus a more specific visualization technique is required. The TEM image of ZnO@Fe3O4 in Fig. 4D clearly demonstrates that Fe3O4 coats the surface of ZnO and that ZnO is complete and did not incur any damage during the coating process.
Optimization of extraction conditions
| Source | Sum of squares | Degrees of freedom | F-value | P-value |
|---|---|---|---|---|
| Factor R | 5143.98 | 4 | 123.82 | 0.000 |
| Factor V | 1050.60 | 4 | 25.29 | 0.000 |
| Factor A | 10.02 | 4 | 0.24 | 0.910 |
The F variance ratio in ANOVA is the ratio of variance due to the effect of a particular factor and can be used to provide a statistical level of significance. In this study, if the F variance ratio is greater than F0.05 (F0.05 = 3.84), then the corresponding factor is considered to make a significant contribution to the overall results. The table shows that factors R and V were significant in the handling of the tea infusion samples. The order of the factors determined in ANOVA was R > V > M, which verifies the results in the mean effect plots and may be due to the fact that only a small quantity of ZnO@Fe3O4 was needed to sufficiently adsorb the extractant.
The optimum extraction conditions for the analytes correspond to the highest average recovery in Fig. 7. From these results, we conclude that the best extraction performance was achieved using 50 mg of the 1% ZnO@Fe3O4–IL (w/w) nanofluid as the extractant with magnetic retrieval using 15 mg of ZnO@Fe3O4.
The dispersive solvent volume is also a critical factor in the microextraction process that affects the volume of the sedimented phase. The effect of the dispersive solvent volume was investigated using different volumes of methanol (150, 200, 250, 300 and 350 μL) containing 50 mg of the IL-based nanofluid. From the results shown in Fig. S3,† we found that the recoveries decreased as the volume of methanol increased. The required volume of the dispersive solvent is very small compared with other DLLME methods, which might be due to the mass transfer effect from the nanoparticles in the extraction solvent. In addition, the lower recoveries obtained with an excessive volume of the dispersive solvent were caused by the increasing solubility of the target compounds in water. Thus, 150 μL was chosen as the optimum volume of dispersive solvent for the rest of the study.
Acetonitrile was used as the desorption solvent because of its good solubilization of the target analytes. The volume of desorption solvent was evaluated from 40–120 μL. The results are shown in Fig. S4.† The recoveries of the five analytes reached a maximum at 60 μL, which was selected as the volume of desorption solvent.
Method validation
Criteria from the Food and Drug Administration (FDA) were used to investigate the above-described MA-ILNF-DLLME technique.45 Calibration curves were established by analyzing different spiked concentrations in tea infusion samples under the optimized conditions. Good linearity was obtained over a concentration range of 5 to 500 μg L−1, with correlation coefficients (R2) varying from 0.9992 to 0.9998. The limits of detection (LODs) ranged from 0.44 to 1 μg L−1. The repeatability and accuracy of this method were evaluated in samples spiked at 50 μg L−1. As shown in Table 2, the intra-day and inter-day RSDs varied from 2.6% to 4.1% and from 4.8% to 7.4%, respectively. The enhancement factor (EF), defined as the ratio of the concentration in the collected phase and that in the sample solution, ranged from 284 to 336. The mean recoveries ranged from 83.0 to 104.4%. These results are satisfactory and within the acceptable range of 70–120%.| Acaricides | Linear equation | Linearity (μg L−1) | R2 | Intra-day precisionb (RSD, %) | Inter-day precisionc (RSD, %) | Enrichment factors | LOD (μg L−1) | Recovery (%) |
|---|---|---|---|---|---|---|---|---|
| a Recovery was calculated from the concentration at 50 μg L−1.b Method precision within a day at spiking with 50 μg L−1 (for n = 3).c Method precision among three days at spiking with 50 μg L−1 (for n = 3). LOD: limits of detection (S/N = 3). | ||||||||
| Clofentezine | Y = 3.41x + 3.12 | 5–500 | 0.9998 | 4.1 | 7.4 | 290 | 0.65 | 103.7 |
| Fenpyroximate | Y = 3.10x + 2.38 | 5–500 | 0.9997 | 2.6 | 5.7 | 336 | 1.00 | 104.4 |
| Diafenthiuron | Y = 2.50x + 5.00 | 5–500 | 0.9992 | 4.5 | 4.4 | 316 | 0.44 | 90.0 |
| Pyridaben | Y = 2.96x − 6.76 | 5–500 | 0.9992 | 2.8 | 6.4 | 284 | 0.65 | 83.0 |
| Spirodiclofen | Y = 2.20x − 8.34 | 5–500 | 0.9992 | 3.1 | 4.8 | 344 | 0.71 | 94.0 |
Application to real samples
The proposed MA-ILNF-DLLME method was successfully applied for the determination of five acaricides in different tea infusion samples (Tie Guanyin, Huoshan Huangya, Longjing tea and Maojian tea). In blank samples, no target acaricides were detected. Real samples spiked at 50 μg L−1 and 200 μg L−1 were also evaluated. Table 3 shows that recovery of the pesticides from different matrixes varied from 79.2 to 93.1% with RSDs of 0.4 to 5.8%. Hence, the developed method has potential as an alternative technique for the analysis of multiple acaricide residues in aqueous tea samples. Typical chromatograms of the extracts of spiked and non-spiked tea samples are shown in Fig. 10.| Sample | Analyte | Spiked conc. (μg L−1) | Recovery ± RSD (%) | Sample | Analyte | Spiked conc.(μg L−1) | Recovery ± RSD (%) |
|---|---|---|---|---|---|---|---|
| Tie Guanyin | Clofentezine | 50 | 83.2 ± 3.2 | Huoshan Huangya | Clofentezine | 50 | 85.5 ± 2.9 |
| 200 | 81.3 ± 0.7 | 200 | 80.6 ± 1.2 | ||||
| Fenpyroximate | 50 | 91.3 ± 0.4 | Fenpyroximate | 50 | 92.3 ± 3.2 | ||
| 200 | 82.7 ± 4.3 | 200 | 83.7 ± 2.4 | ||||
| Diafenthiuron | 50 | 79.2 ± 2.6 | Diafenthiuron | 50 | 87.9 ± 2.0 | ||
| 200 | 85.7 ± 5.4 | 200 | 87.2 ± 1.2 | ||||
| Pyridaben | 50 | 89.4 ± 3.3 | Pyridaben | 50 | 88.5 ± 2.9 | ||
| 200 | 85.9 ± 0.4 | 200 | 81.4 ± 1.6 | ||||
| Spirodiclofen | 50 | 88.6 ± 2.1 | Spirodiclofen | 50 | 91.5 ± 3.1 | ||
| 200 | 90.1 ± 5.8 | 200 | 93.1 ± 3.4 | ||||
| Long Jing tea | Clofentezine | 50 | 81.6 ± 3.4 | Maojian tea | Clofentezine | 50 | 85.6 ± 1.7 |
| 200 | 80.6 ± 1.2 | 200 | 80.6 ± 1.6 | ||||
| Fenpyroximate | 50 | 91.3 ± 0.4 | Fenpyroximate | 50 | 93.1 ± 4.0 | ||
| 200 | 83.7 ± 2.4 | 200 | 82.7 ± 3.3 | ||||
| Diafenthiuron | 50 | 80.1 ± 3.8 | Diafenthiuron | 50 | 80.4 ± 1.8 | ||
| 200 | 87.2 ± 1.2 | 200 | 89.4 ± 0.6 | ||||
| Pyridaben | 50 | 86.4 ± 3.9 | Pyridaben | 50 | 82.4 ± 2.8 | ||
| 200 | 81.4 ± 1.6 | 200 | 81.2 ± 1.5 | ||||
| Spirodiclofen | 50 | 87.2 ± 1.9 | Spirodiclofen | 50 | 85.8 ± 0.9 | ||
| 200 | 93.1 ± 3.4 | 200 | 90.5 ± 4.7 |
Comparison with other methods
The proposed MA-ILNF-DLLME method for determining acaricides was compared with other published methods, and the results are summarized in Table 4. As observed, the method described herein is faster than most other methods. In sample preparation, extraction equilibrium is a key factor determining the maximum analyte concentration that can be preconcentrated and thus affects the sensitivity of the method. The use of an IL-based nanofluid as the extraction solvent in this method results in rapid attainment of equilibrium due to the large surface area between the aqueous samples and extractant. Additionally, the present work does not require special instrumentation or large volumes of volatile organic solvents.| Analytes | Method | Matrix | Extractant | Extraction time (min) | Amount of organic solvent | LOD (μg L−1) | Reference |
|---|---|---|---|---|---|---|---|
| a UA-IL-DLLME: ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction; UA-ISFME: ultrasound-assisted in situ solvent formation microextraction; TEME: totally organic solvent-free emulsification microextraction; SFE-μLC: supercritical fluid extraction-capillary liquid chromatography. | |||||||
| Clofentezine | UA-IL-DLLME-HPLC | Water samples | [OMIM]PF6 | 7 min | 250 μL of methanol + 60 μL of [OMIM]PF6 + 50 μL of acetonitrile | 0.85–2.15 | 3 |
| Fenpyroximate | |||||||
| Diafenthiuron | |||||||
| Pyridaben | |||||||
| Spirodiclofen | |||||||
| Clofentezine | UA-ISFME-HPLC | Water samples | [OMIM]Cl | 7 min | 40 mg of [OMIM]Cl + 50 μL of acetonitrile | 0.54–1.48 | 3 |
| Fenpyroximate | |||||||
| Diafenthiuron | |||||||
| Pyridaben | |||||||
| Spirodiclofen | |||||||
| Fenpyroximate | TEME-HPLC | Fruit juice | [HMIM]NTF2 | 16 min | 53 μL of [HMIM]NTF2 | 0.02–0.06 | 46 |
| Spirodiclofen | |||||||
| Fenpyroximate | SFE-μLC-UV | Apple sample | CO2 gas | >41 min | >13 mL of acetonitrile | 0.02–11.1 | 47 |
| Clofentezine | SPE-d-SPE-HPLC | Olive oil | SPE | >15 min | >28 mL of acetonitrile | 0.26 | 48 |
| Five acaricides | MA-ILNF-DLLME | Tea infusion | Nanofluid | 2 min | 150 μL of methanol + 50 μL of [OMMIM]NTF2 + 60 μL of acetonitrile | 0.02–0.1 | In this work |
Conclusion
We proposed a sensitive MA-ILNF-DLLME method to determine trace levels of acaricides in tea infusions in combination with HPLC. In this study, a novel type of magnetic nanoparticle, ZnO@Fe3O4, was synthesized, and a novel nanofluid was prepared by dispersing the prepared nanoparticles in [OMIMM]NTF2. This IL-based nanofluid was used as an extractant in this microextraction approach. The nanofluid is magnetically absorbed by ZnO@Fe3O4 to allow separation without centrifugation. The merits of this method include the ability to use IL-based nanofluids, which are relatively safe and green, to replace organic solvents in DLLME; extractant retrieval assisted by the nanoparticles in the nanofluid to enhance the magnetic property of the extraction solution and shorten the extraction time; and acceptable recovery values, which indicate the method's high utility. In summary, this sample preparation method is rapid, simple, timesaving, efficient, sensitive and environmentally friendly, and it has great potential when applied for the determination of pesticides.Acknowledgements
Supported by the fund of the National Natural Science Foundation of China (Project no. 21507159, 21277172 and 21377163) and the Chinese Universities Scientific Fund (Project no. 2016QC082).References
- B. Kanrar, S. Mandal and A. Bhattacharyya, J. Chromatogr. A, 2010, 1217, 1926–1933 CrossRef CAS PubMed.
- C. Cabrera, R. Artacho and R. Giménez, J. Am. Coll. Nutr., 2006, 25, 79–99 CrossRef CAS PubMed.
- B. Peng, X. Yang, J. Zhang, F. Du, W. Zhou, H. Gao and R. Lu, J. Sep. Sci., 2013, 36, 2196–2202 CrossRef CAS PubMed.
- X. Zhang, F. Luo, Z. Lou, M. Lu and Z. Chen, J. Chromatogr. A, 2014, 1359, 212–223 CrossRef CAS PubMed.
- http://www.ec.europa.eu/food/plant/pesticides/eu-pesticides-database.
- K. B. Borges, E. F. Freire, I. Martins and M. E. de Siqueira, Talanta, 2009, 78, 233–241 CrossRef CAS PubMed.
- A. L. Saber, Talanta, 2009, 78, 295–299 CrossRef CAS PubMed.
- W. Ahmad, A. A. Al-Sibaai, A. S. Bashammakh, H. Alwael and M. S. El-Shahawi, TrAC, Trends Anal. Chem., 2015, 72, 181–192 CrossRef CAS.
- A. Gałuszka, Z. Migaszewski and J. Namieśnik, TrAC, Trends Anal. Chem., 2013, 50, 78–84 CrossRef.
- J. W. Yoon, J. S. Kim, T. H. Kim, Y. J. Hong, Y. C. Kang and J. H. Lee, Small, 2016, 12, 4229–4240 CrossRef CAS PubMed.
- P. Giungato, G. de Gennaro, P. Barbieri, S. Briguglio, M. Amodio, L. de Gennaro and F. Lasigna, J. Cleaner Prod., 2016, 133, 1395–1402 CrossRef CAS.
- Y. Wang, X. Hua and H. Yu, Analyst, 2016, 141, 4647–4653 RSC.
- L. Petti, N. Münzenrieder, C. Vogt, H. Faber, L. Büthe, G. Cantarella, F. Bottacchi, T. D. Anthopoulos and G. Tröster, Appl. Phys. Rev., 2016, 3, 021303 Search PubMed.
- J. Nawrocki, C. Dunlap, A. McCormick and P. W. Carr, J. Chromatogr. A, 2004, 1028, 1–30 CrossRef CAS PubMed.
- S. A. Olushola, S. F. Olalekan, A. A. Folahan, F. P. Leslie and B. J. Ximba, Water SA, 2014, 40, 659 CrossRef.
- N. Zhang, J. Gao, C. Huang, W. Liu, P. Tong and L. Zhang, Anal. Chim. Acta, 2016, 934, 122–131 CrossRef CAS PubMed.
- S. Mandal and S. Natarajan, J. Environ. Chem. Eng., 2015, 3, 1185–1193 CrossRef CAS.
- J. Saien, H. Bamdadi and S. Daliri, J. Ind. Eng. Chem., 2015, 21, 1152–1159 CrossRef CAS.
- L. R. Mason, D. Ciceri, D. J. E. Harvie, J. M. Perera and G. W. Stevens, Microfluid. Nanofluid., 2012, 14, 197–212 CrossRef.
- S. Basu and A. Miglani, Int. J. Heat Mass Transfer, 2016, 96, 482–503 CrossRef CAS.
- S.-S. Ashrafmansouri and M. Nasr Esfahany, Sep. Purif. Technol., 2015, 151, 74–81 CrossRef CAS.
- S. Shim, J. Shim, W. R. Taylor, F. Kosari, G. Vasmatzis, D. A. Ahlquist and R. Bashir, Biomed. Microdevices, 2016, 18, 28 CrossRef PubMed.
- A. Vahedi, A. Molaei Dehkordi and F. Fadaei, AIChE J., 2016, 62, 4466–4479 CrossRef CAS.
- H. Hu, B. Liu, J. Yang, Z. Lin and W. Gan, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1027, 200–206 CrossRef CAS PubMed.
- M. Wang, W. Bi, X. Huang and Y. Chen Dda, J. Chromatogr. A, 2016, 1449, 8–16 CrossRef CAS PubMed.
- P. Sun and D. W. Armstrong, Anal. Chim. Acta, 2010, 661, 1–16 CrossRef CAS PubMed.
- M. J. Trujillo-Rodríguez, P. Rocío-Bautista, V. Pino and A. M. Afonso, TrAC, Trends Anal. Chem., 2013, 51, 87–106 CrossRef.
- L. Hu, P. Zhang, R. Lu, W. Zhou and H. Gao, Anal. Methods, 2013, 5, 5376–5385 RSC.
- A. F. M. Cláudio, M. G. Freire and J. A. P. Coutinho, Green Chem., 2013, 15, 2002–2010 RSC.
- M. Asensio-Ramos, J. Hernandez-Borges, T. M. Borges-Miquel and M. A. Rodriguez-Delgado, J. Chromatogr. A, 2011, 1218, 4808–4816 CrossRef CAS PubMed.
- M. M. Vazquez, P. P. Vazquez, M. M. Galera and A. U. Moreno, J. Chromatogr. A, 2014, 1356, 1–9 CrossRef CAS PubMed.
- P. Zhang, Y. Li, B. Peng, S. Li, H. Gao and W. Zhou, Anal. Methods, 2013, 5, 2241–2248 RSC.
- H. Z. Song, Z. Q. Luo, C. Z. Wang, X. F. Hao and J. G. Gao, Carbohydr. Polym., 2013, 98, 161–167 CrossRef CAS PubMed.
- M. Amde, J. F. Liu, Z. Q. Tan and D. Bekana, Talanta, 2016, 149, 341–346 CrossRef CAS PubMed.
- M. Amde, Z. Q. Tan, R. Liu and J. F. Liu, J. Chromatogr. A, 2015, 1395, 7–15 CrossRef CAS PubMed.
- M. I. Leong and S. D. Huang, J. Chromatogr. A, 2008, 1211, 8–12 CrossRef CAS PubMed.
- D. L. Giokas, Q. Zhu, Q. Pan and A. Chisvert, J. Chromatogr. A, 2012, 1251, 33–39 CrossRef CAS PubMed.
- Z.-G. Shi and H. K. Lee, Anal. Chem., 2010, 82, 1540–1545 CrossRef CAS PubMed.
- T. Zhang, J. F. Guo, L. Bai, Z. G. Shi and L. M. Qi, J. Liq. Chromatogr. Relat. Technol., 2014, 38, 104–110 CrossRef.
- J. Zhang, M. Li, M. Yang, B. Peng, Y. Li, W. Zhou, H. Gao and R. Lu, J. Chromatogr. A, 2012, 1254, 23–29 CrossRef CAS PubMed.
- O. Russina, M. Beiner, C. Pappas, M. Russina, V. Arrighi, T. Unruh, C. L. Mullan, C. Hardacre and A. Triolo, J. Phys. Chem. B, 2009, 113, 8469–8474 CrossRef CAS PubMed.
- J. Cao, W. Fu, H. Yang, Q. Yu, Y. Zhang, S. Wang, H. Zhao, Y. Sui, X. Zhou, W. Zhao, Y. Leng, H. Zhao, H. Chen and X. Qi, Mater. Sci. Eng., B, 2010, 175, 56–59 CrossRef CAS.
- X.-Q. Wang and A. S. Mujumdar, Int. J. Therm. Sci., 2007, 46, 1–19 CrossRef.
- H.-J. Pan and W.-H. Ho, Anal. Chim. Acta, 2004, 527, 61–67 CrossRef CAS.
- U.S. FDA, Guidance for Industry (draft): Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls and Documentation, 2000 Search PubMed.
- J. Zhang, Z. Liang, H. Guo, P. Gao, R. Lu, W. Zhou, S. Zhang and H. Gao, Talanta, 2013, 115, 556–562 CrossRef CAS PubMed.
- B. L. Halvorsen, C. Thomsen, T. Greibrokk and E. Lundanes, J. Chromatogr. A, 2000, 880, 121–128 CrossRef CAS PubMed.
- T. Tuzimski and T. Rejczak, Food Chem., 2016, 190, 71–79 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22353b |
| This journal is © The Royal Society of Chemistry 2016 |