Copper-catalyzed carbonylation of anilines by diisopropyl azodicarboxylate for the synthesis of carbamates†
Muhammad Usman,
Zhi-Hui Ren,
Yao-Yu Wang and
Zheng-Hui Guan*
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, Department of Chemistry & Materials Science Northwest University, Xi'an 710127, P. R. China. E-mail: guanzhh@nwu.edu.cn; Web: http://www.chem.nwu.edu.cn/teacher.php?id=189
First published on 27th October 2016
Abstract
A Cu-catalyzed efficient methodology for the direct carbonylation of anilines has been developed. The N–H bond cleavage and N–C bond formation were notably achieved under solvent-free conditions and a variety of carbamates were synthesized from readily available anilines using diisopropyl azodicarboxylate (DIAD) as the carbonylating source.
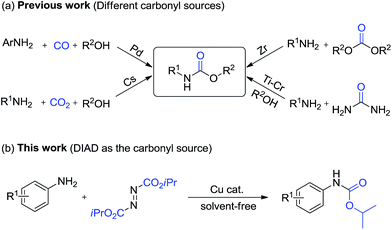
Dialkyl azodicarboxylates are very useful reagents in organic synthesis such as for the Mitsunobu reaction.1 Due to its unique structural and electronic features, azodicarboxylates are used in reactions proceeding via a zwitterionic intermediate,2 oxidation,3 aldehydic C–H bond functionalization via hydroacylation,4 amination of C–H bonds,5 and α-amination of carbonyl and cyanoacetate compounds.6 However, the study of dialkyl azodicarboxylates as carbonylating reagent is very limited.7
Organic carbamates are important intermediates for the synthesis of pesticides, herbicides and pharmaceutical drugs.8 Several methods for carbamate synthesis have been reported using N,N′-carbonyldiimidazole, carbamoylimidazolium salts and toxic phosgene or its derivatives.8c,9 The other available carbonyl sources for the synthesis of carbamates are urea, dialkyl carbonate, CO2 and CO (Scheme 1a).10 Transition metal-catalyzed oxidative11 and reductive12 carbonylation of amines and nitro compounds with poisonous CO have been reported, respectively. Industrially important methods are based on the carbonylation of amines with organic carbonates.13 The synthesis of carbamates has also been accomplished via Lossen, Curtius and Hofmann rearrangements.14 The limitations of these methods are due to the multi-step procedures, harsh reaction conditions and lack of easily available substrates. Despite some advances, the development of more convenient and alternative methods based on other carbonyl sources is still desirable (Scheme 1b). As part of our previous work related to carbamate synthesis,10d we speculated that carbamates could be formed with dialkyl azodicarboxylate under mild reaction conditions. We selected copper as a possible catalyst because of its robustness.15 Cu-catalyzed direct C–N bond formation is a more desirable atom-economic synthetic approach.15a In this regard, Cu-catalyzed reactions with diazo compounds have gained significant achievement.15c However, to the best of our knowledge, copper catalysts have never been studied in a carbonylation reaction with dialkyl azodicarboxylates. Herein, we wish to report a simple method for the synthesis of carbamates via a novel reaction using diisopropyl azodicarboxylate (DIAD) as the carbonyl source over copper-catalyst and notably under solvent-free conditions.
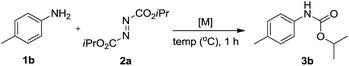
We initially tried to optimize the reaction conditions of aniline 1b with DIAD 2a using different solvents. Gratifyingly, we observed that 1b could be transformed into the desired carbamate 3b in 23% yield (Table 1, entry 1) using CuI as the catalyst in THF. In addition, the effects of various organic solvents and catalysts were investigated, and we found that solvents do not play an important role in this reaction. When toluene, DMSO or DMF was used as a solvent, there was no major change in carbonylation and a very low yield for the corresponding product was obtained (15–20%) (Table 1, entries 2–4). Changing the catalysts in THF further reduced the product yield (Table 1, entries 5–7). When Pd(OAc)2 was used as a catalyst, no reaction was observed (Table 1, entry 8). Moreover, we speculated that the reaction yield could improve in solvent-free conditions. Delightedly, in the absence of a solvent, the yield for 3a increased to 27% when the reaction was carried out in the presence of CuI at room temperature for 1 h (Table 1, entry 9). The best result was obtained when the reaction was performed in the presence of Cu(OAc)2 (Table 1, entry 11). Changing the catalyst loading showed less efficiency in terms of yield (Table 1, entries 12 and 13). Furthermore, no reaction occurred in the absence of a copper catalyst (Table 1, entry 14).
| Entry | Catalyst | Solvent | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1b (0.3 mmol), 2a (0.6 mmol) and catalyst (5 mol%) in solvent (1 ml) under an Ar atmosphere for 1 h.b Isolated yields.c Cu(OAc)2 (1 mol%).d Cu(OAc)2 (20 mol%). | ||||
| 1 | CuI | THF | 120 | 23 |
| 2 | CuI | Toluene | 120 | 15 |
| 3 | CuI | DMSO | 120 | 20 |
| 4 | CuI | DMF | 120 | 18 |
| 5 | CuBr2 | THF | 120 | 19 |
| 6 | CuCl2 | THF | 120 | Trace |
| 7 | Cu(NO3)2 | THF | 120 | Trace |
| 8 | Pd(OAc)2 | THF | 120 | 0 |
| 9 | CuI | — | RT | 27 |
| 10 | Cu(TFA)2 | — | RT | 21 |
| 11 | Cu(OAc)2 | — | RT | 75 |
| 12c | Cu(OAc)2 | — | RT | 66 |
| 13d | Cu(OAc)2 | — | RT | 68 |
| 14 | — | — | RT | 0 |
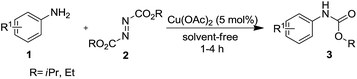
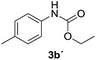
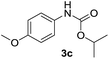
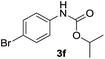
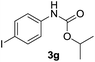
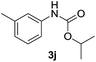
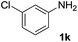
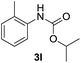
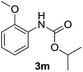
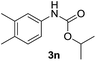
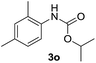
After optimizing the reaction conditions, the substrate scope of the reaction was investigated (Table 2). This solvent free carbonylation was found to be a good methodology for the synthesis of carbamates. Carbonylation of aniline 1a with DIAD 2a gives carbamate 3a in 81% yield (Table 2, entry 1). Likewise, diethyl azodicarboxylate (DEAD) 2b led to comparatively the same yield (Table 2, entry 2). Anilines substituted at the aryl ring with Me, MeO, F, Cl Br, and I were efficiently converted into the corresponding products (Table 2, entries 3–9). However, the reaction of 1h and 1i was sluggish and reached partial completion only after long reaction times at elevated temperatures (Table 2, entries 10 and 11). When meta-substituted anilines 1j and 1k were employed, the corresponding products 3j and 3k were obtained in up to 71% and 81% yield, respectively (Table 2, entries 12 and 13). In addition, ortho-substituted anilines 1l and 1m were transformed into the corresponding carbamates 3l and 3m in moderate yields (Table 2, entries 14 and 15). Moreover, 3,4-dimethylaniline and 2,4-di-methylaniline smoothly furnished the desired products in good yields (Table 2, entries 16 and 17). Interestingly, when the heterocyclic analogue 1p was subjected to the standard reaction conditions, the desired product 3p was obtained in 30% yield (Table 2, entry 18). However, the scope of this reaction was limited to primary anilines.
| Entry | Substrate | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: anilines 1 (0.3 mmol), 2 (0.6 mmol) and Cu(OAc)2 (5 mol%) under an Ar atmosphere at room temperature for 1 h.b Isolated yields.c 80 °C instead of room temperature for 4 h.d 80 °C for 1 h. R = iPr, Et. | |||
| 1 |  |
 |
81 |
| 2 |  |
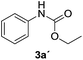 |
79 |
| 3 | 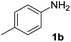 |
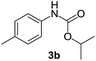 |
75 |
| 4 | 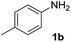 |
 |
74 |
| 5 | 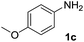 |
 |
74 |
| 6 | 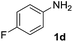 |
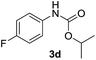 |
78 |
| 7 | 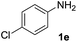 |
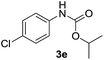 |
83 |
| 8 | 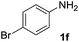 |
 |
82 |
| 9 | 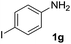 |
 |
66 |
| 10 | 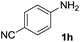 |
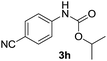 |
48c |
| 11 | 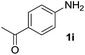 |
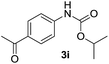 |
52c |
| 12 | 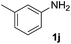 |
 |
71 |
| 13 | 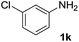 |
 |
81 |
| 14 | 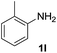 |
 |
53 |
| 15 | 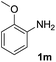 |
 |
45 |
| 16 | 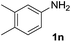 |
 |
79 |
| 17 | 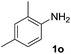 |
 |
73 |
| 18 | 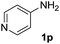 |
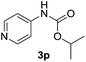 |
30d |
In order to demonstrate the synthetic utility of this method, a further transformation of DEAD 2b was conducted with benzimidazole 1q (Scheme 2). To our delight, we found that carbonylation of the N–H functionality in 1q proceeded smoothly in a highly selective manner at elevated temperature (80 °C) for 1 h to afford the corresponding product 3q in 78% yield.
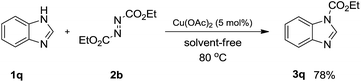 | ||
| Scheme 2 Synthetic transformation of 1q. Reaction conditions: 1q (0.3 mmol), DEAD 2b (0.6 mmol) and Cu(OAc)2 (5 mol%) under an Ar atmosphere at 80 °C for 1 h. | ||
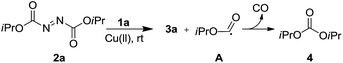
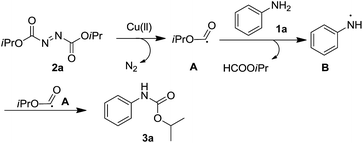
According to the proposed mechanism for the reaction, the Cu-catalyzed carbon and heteroatom bond formation occurs through a radical pathway.15a The high yields obtained for electron-deficient anilines are consistent with the higher stability of electron-deficient aniline radicals when compared with electron-rich aniline radicals.16 Furthermore, the decomposition of the aliphatic azo compound afforded a free radical that could be used for further transformations.7a,7b,17 We found that when 1a reacted with 2a under standard reaction conditions, 3a and diisopropyl carbonate 4 were formed (Scheme 3).7a We believe that decomposition of 2a would generate the oxyacyl radical A,18 which would either react with anilines to form carbamates or would undergo decarbonylation to generate diisopropyl carbonate.
On the basis of these studies, a possible mechanism for the C–N bond formation via reaction of DIAD with aniline is proposed (Scheme 4). For the N–H bond carbonylation using DIAD, the proposed mechanism is triggered by the copper catalyst; DIAD 2a decomposes to give the oxyacyl radical A, which is believed to abstract one hydrogen from aniline 1a to generate the aminyl radical B.19 DIAD 2a serves as an aniline radical initiator as well as a radical trapping reagent in the reaction.20 The resulting A and B radicals combine to give the corresponding carbamate 3a.
In conclusion, we discovered a novel method for the synthesis of carbamates by a Cu-catalyzed reaction of simple anilines with commercially available diethyl or diisopropyl azodicarboxylate. The N–H bond cleavage and N–C bond formation was notably achieved under solvent-free conditions. Various electron-donating and electron-withdrawing groups on the anilines are compatible with this method. The use of readily available dialkyl azodicarboxylate and an inexpensive copper catalyst makes this a mild, general and efficient method, thus providing an extremely preferable protocol for the synthesis of a variety of carbamates. Further studies on extending the scope of the reaction and a detailed investigation into the reaction mechanism are under progress in our laboratory.
Acknowledgements
This study was supported by the generous grants from the National Natural Science Foundation of China (21272183, 21622203 and 21472147) and the Fund of the Shanxi Province (2016JM2007).Notes and references
- (a) R. Dembinski, Eur. J. Org. Chem., 2004, 2763 CrossRef CAS; (b) K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. Kumar, Chem. Rev., 2009, 109, 2551 CrossRef CAS PubMed; (c) D. Camp, M. V. Itzstein and I. D. Jenkins, Tetrahedron, 2015, 71, 4946 CrossRef CAS; (d) B. H. Lipshutz, D. W. Chung, B. Rich and R. Corral, Org. Lett., 2006, 8, 5069 CrossRef CAS PubMed; (e) R. D. Otte, T. Sakata, I. A. Guzei and D. Lee, Org. Lett., 2005, 7, 495 CrossRef CAS PubMed; (f) D. P. Furkert, B. Breitenbach, L. Juen, I. Sroka, M. Pantin and M. A. Brimble, Eur. J. Org. Chem., 2014, 7806 CrossRef CAS.
- (a) V. Nair, A. T. Biju, K. Mohanan and E. Suresh, Org. Lett., 2006, 8, 2213 CrossRef CAS PubMed; (b) V. Nair, A. T. Biju, A. U. Vinod and E. Suresh, Org. Lett., 2005, 7, 5139 CrossRef CAS PubMed; (c) V. Nair, A. T. Biju, K. G. Abhilash, R. S. Menon and E. Suresh, Org. Lett., 2005, 7, 2121 CrossRef CAS PubMed; (d) D. Hong, Y. Zhu, X. Lin and Y. Wang, Tetrahedron, 2011, 67, 650 CrossRef CAS; (e) V. Nair, S. C. Mathew, A. T. Biju and E. Suresh, Tetrahedron Lett., 2007, 48, 9018 CrossRef CAS.
- (a) X. Xu, X. Li, L. Ma, N. Ye and B. Weng, J. Am. Chem. Soc., 2008, 130, 14048 CrossRef CAS PubMed; (b) X. Xu and X. Li, Org. Lett., 2009, 11, 1027 CrossRef CAS PubMed; (c) M. Hayashi, M. Shibuya and Y. Iwabuchi, J. Org. Chem., 2012, 77, 3005 CrossRef CAS PubMed; (d) N. Iranpoor, H. Firouzabadi, D. Khalili and R. Shahin, Tetrahedron Lett., 2010, 51, 3508 CrossRef CAS; (e) H. T. Cao and R. Grée, Tetrahedron Lett., 2009, 50, 1493 CrossRef CAS.
- (a) H.-B. Zhang, Y. Wang, Y. Gu and P.-F. Xu, RSC Adv., 2014, 4, 27796 RSC; (b) Y. Qin, Q. Peng, J. Song and D. Zhou, Tetrahedron Lett., 2011, 52, 5880 CrossRef CAS; (c) A. Mariappan, K. Rajaguru, S. Muthusubramanian and N. Bhuvanesh, Tetrahedron Lett., 2015, 56, 338 CrossRef CAS; (d) D. Lee and R. D. Otte, J. Org. Chem., 2004, 69, 3569 CrossRef CAS PubMed.
- (a) B. Qian, L. Yang and H. Huang, Tetrahedron Lett., 2013, 54, 711 CrossRef CAS; (b) Y. Wang, B. Frett, N. McConnell and H.-Y. Li, Org. Biomol. Chem., 2015, 13, 2958 RSC; (c) S. M. Inamdar, V. K. More and S. K. Mandal, Tetrahedron Lett., 2013, 54, 530 CrossRef CAS; (d) V. A. Schmidt and E. J. Alexanian, J. Am. Chem. Soc., 2011, 133, 11402 CrossRef CAS PubMed; (e) G. Huang, Y. Xia, C. Sun, J. Li and D. Lee, J. Org. Chem., 2013, 78, 988 CrossRef CAS PubMed.
- (a) X. Liu, H. Li and L. Deng, Org. Lett., 2005, 7, 167 CrossRef CAS PubMed; (b) T. Mashiko, K. Hara, D. Tanaka, Y. Fujiwara, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2007, 129, 11342 CrossRef CAS PubMed; (c) C. Thomassigny, D. Prim and C. Greck, Tetrahedron Lett., 2006, 47, 1117 CrossRef CAS.
- (a) W.-Y. Yu, W. N. Sit, K.-M. Lai, Z. Zhou and A. S. C. Chan, J. Am. Chem. Soc., 2008, 130, 3304 CrossRef CAS PubMed; (b) Y. Huang, G. Li, J. Huang and J. You, Org. Chem. Front., 2014, 1, 347 RSC; (c) S. Ramesh, P. N. Arunachalam and A. Lalitha, RSC Adv., 2013, 3, 8666 RSC.
- (a) P. Adams and F. A. Baron, Chem. Rev., 1965, 65, 567 CrossRef CAS; (b) T.-T. Wu, J. Huang, N. D. Arrington and G. M. Dill, J. Agric. Food Chem., 1987, 35, 817 CrossRef CAS; (c) D. Chaturvedi, N. Mishra and V. Mishra, Curr. Org. Synth., 2007, 4, 308 CrossRef CAS; (d) G. Xiaoguang, S. Jianpeng, L. Jian, W. Liguo, M. Yubo, S. Feng and D. Youquan, Chin. J. Chem., 2010, 28, 164 CrossRef.
- (a) L. Cotarca, P. Delogu, A. Nardelli and V. Sunjic, Synthesis, 1996, 553 CrossRef CAS; (b) H. Eckert and B. Forster, Angew. Chem., Int. Ed. Engl., 1987, 26, 894 CrossRef; (c) Z.-H. Fu and Y. Ono, J. Mol. Catal., 1994, 91, 399 CrossRef CAS; (d) H. Babad and A. G. Zeiler, Chem. Rev., 1973, 73, 75 CrossRef CAS; (e) D. D'Addona and C. G. Bochet, Tetrahedron Lett., 2001, 42, 5227 CrossRef; (f) J. A. Grzyb, M. Shen, C. Yoshina-Ishii, W. Chi, R. S. Brown and R. A. Batey, Tetrahedron, 2005, 61, 7153 CrossRef CAS.
- (a) P. Wang, Y. Ma, S. Liu, F. Zhou, B. Yang and Y. Deng, Green Chem., 2015, 17, 3964 RSC; (b) C. Han and J. A. Porco, Org. Lett., 2007, 9, 1517 CrossRef CAS PubMed; (c) A. Ion, C. V. Doorslaer, V. Parvulescu, P. Jacobs and D. D. Vos, Green Chem., 2008, 10, 111 RSC; (d) Z.-H. Guan, H. Lie, M. Chen, Z.-H. Ren, Y. Bai and Y.-Y. Wang, Adv. Synth. Catal., 2012, 354, 489 CrossRef CAS.
- (a) S. Fukuoka, M. Chono and M. Kohno, J. Chem. Soc., Chem. Commun., 1984, 399 RSC; (b) S. Fukuoka, M. Chono and M. Kohno, J. Org. Chem., 1984, 49, 1458 CrossRef CAS; (c) S. P. Gupte and R. V. Chaudhari, J. Catal., 1988, 114, 246 CrossRef CAS; (d) K.-T. Li and Y.-J. Peng, J. Catal., 1993, 143, 631 CrossRef CAS; (e) H. Alper and F. W. Hartstock, J. Chem. Soc., Chem. Commun., 1985, 1141 RSC; (f) B. Wan, S. Liao and D. Yu, Appl. Catal., A, 1999, 183, 81 CrossRef CAS.
- (a) S. Cenini, C. Crotti, M. Pizzotti and F. Porta, J. Org. Chem., 1988, 53, 1243 CrossRef CAS; (b) F. Ragaini and S. Cenini, Organometallics, 1994, 13, 1178 CrossRef CAS; (c) F. Paul, J. Fischer, P. Ochsenbein and J. A. Osborn, Organometallics, 1998, 17, 2199 CrossRef CAS; (d) A. M. Tafesh and J. Weiguny, Chem. Rev., 1996, 96, 2035 CrossRef CAS PubMed.
- (a) M. Selva, P. Tundo and A. Perosa, Tetrahedron Lett., 2002, 43, 1217 CrossRef CAS; (b) M. Curini, F. Epifano, F. Maltese and O. Rosati, Tetrahedron Lett., 2002, 43, 4895 CrossRef CAS; (c) T. Sima, S. Guo, F. Shi and Y. Deng, Tetrahedron Lett., 2002, 43, 8145 CrossRef CAS; (d) T. Baba, M. Fujiwara, A. Oosaku, A. Kobayashi, R. G. Deleon and Y. One, Appl. Catal., A, 2002, 227, 1 CrossRef CAS; (e) I. Vauthey, F. Valot, C. Gozzi, F. Fache and M. Lemaire, Tetrahedron Lett., 2000, 41, 6347 CrossRef CAS; (f) E. Reixach, R. M. Haak, S. Wershofen and A. Vidal-Ferran, Ind. Eng. Chem. Res., 2012, 51, 16165 CrossRef CAS; (g) Y. Wang and B. Liu, Catal. Sci. Technol., 2015, 5, 109 RSC.
- (a) D. Chaturvedi, Tetrahedron, 2012, 68, 15 CrossRef CAS; (b) S. Yoganathan and S. J. Miller, Org. Lett., 2013, 15, 602 CrossRef CAS PubMed and reference cited therein; (c) E. V. Vinogradova, N. H. Park, B. P. Fors and S. L. Buchwald, Org. Lett., 2013, 15, 1394 CrossRef CAS PubMed and reference cited therein.
- (a) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC; (b) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed; (c) X. Zhao, Y. Zhang and J. Wang, Chem. Commun., 2012, 48, 10162 RSC.
- Z. Li and J.-P. Cheng, J. Org. Chem., 2003, 68, 7350 CrossRef CAS PubMed.
- (a) H. Xu, P.-T. Liu, Y.-H. Li and F.-S. Han, Org. Lett., 2013, 15, 3354 CrossRef CAS PubMed; (b) D. Zhou, Z.-H. Li, J. Li, S.-H. Li, M.-W. Wang, X.-L. Luo, G.-L. Ding, R.-L. Sheng, M.-J. Fu and S. Tang, Eur. J. Org. Chem., 2015, 1606 CrossRef CAS; (c) Y. Xie, S. Guo, L. Wu, C. Xia and H. Huang, Angew. Chem., Int. Ed., 2015, 54, 5900 CrossRef CAS PubMed.
- (a) V. Malatesta and K. U. Ingod, Tetrahedron Lett., 1973, 14, 3311 CrossRef; (b) A. Alberti and A. Hudson, Tetrahedron Lett., 1982, 23, 453 CrossRef CAS; (c) B. P. Roberts and J. N. Winter, Tetrahedron Lett., 1979, 20, 3575 CrossRef; (d) P. Stilbs, G. Ahlgren and B. Åkermark, Tetrahedron Lett., 1972, 13, 2387 CrossRef.
- Stable aminyl radicals are generally prepared by dehydrogenation by oxidants. While, the utility of Ag2O and PbO2 with reduced amount of 2a does not work to save one equivalent of DIAD rather gives a trace level of product and low yield along with complex reaction mixture, respectively. see: W. C. Danen and F. A. Neugebauer, Angew. Chem., Int. Ed., 1975, 14, 783 CrossRef.
- (a) X.-Y. Duan, X.-L. Yang, R. Fang, X.-X. Peng, W. Yu and B. Han, J. Org. Chem., 2013, 78, 10692 CrossRef CAS PubMed; (b) B. Han, X.-L. Yang, R. Fanf, W. Yu, C. Wang, X.-Y. Duan and S. Liu, Angew. Chem., Int. Ed., 2012, 51, 8816 CrossRef CAS PubMed and reference cited therein.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, characterization of all compounds and copies of 1H and 13C NMR spectra for all selected compounds. See DOI: 10.1039/c6ra22108d |
| This journal is © The Royal Society of Chemistry 2016 |