Structure, and thermal and mechanical properties of poly(propylene carbonate) capped with different types of acid anhydride via reactive extrusion
Abstract
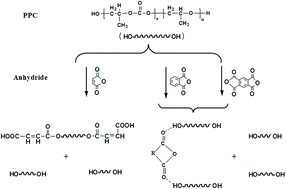
Three types of anhydride (maleic anhydride (MA), phthalic anhydride (PA) and pyromellitic dianhydride (PMDA)) were melt blended to end-cap poly(propylene carbonate) (PPC) by reactive extrusion. The effect of anhydride types on thermal, rheological, and mechanical properties of PPC was investigated. Results of FTIR spectra, Raman spectra and GPC indicate that the reaction mechanism between PPC with MA was different from PPC with PA and PMDA. During the extrusion processing in the presence of MA, end capping occurs for the free polymer ends and reduces chain depolymerization in a conventional way. For PA and PMDA, some non-covalent interaction may exist and it is deduced that hydrogen bonding may occur. In addition, TGA results show that the thermal degradation temperature of PPC could be improved by adding three types of anhydride, and the T−5% of PPC–PMDA was the highest and increased by 26.3 °C. The tensile strength and Young's modulus of PPC is also improved with the addition of MA, PA and PMDA. Moreover, the value of elongation at break of all end-capped PPC still can remain higher than 1000%.

 Please wait while we load your content...
Please wait while we load your content...