Cross-linked porous α-Fe2O3 nanorods as high performance anode materials for lithium ion batteries
Yuxuan Zhua,
Qinghong Wang*a,
Xinsheng Zhaob and
Boyu Yuanb
aSchool of Chemistry and Chemical Engineering, Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, Jiangsu Normal University, Xuzhou, Jiangsu 221116, PR China. E-mail: wangqh@jsnu.edu.cn
bHydrogen Energy Laboratory, School of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou, Jiangsu 221116, China
First published on 4th October 2016
Abstract
Novel cross-linked porous α-Fe2O3 nanorods are synthesized via a facile hydrothermal-calcination method. For comparison, Fe2O3 nanoparticles are prepared. The as-prepared α-Fe2O3 nanorods are evaluated as anode materials for lithium ion batteries. Electrochemical measurements reveal that the cross-linked porous α-Fe2O3 nanorods display a high initial discharge capacity of 1285.2 mA h g−1 at 0.2C and it still remains 740.2 mA h g−1 after 300 cycles, which are much higher than those of the Fe2O3 nanoparticles. Moreover, the cross-linked porous electrode delivers excellent cycle stability at a high rate density (about 600 mA h g−1 at 1C and 520 mA h g−1 at 2C after 300 cycles). The improved electrochemical properties may be attributed to the regular 1D nanostructure, the cross-linked nanostructure and the abundant pores inlaid in the nanorods of the as-prepared α-Fe2O3.
Introduction
Transition metal oxides are considered to be potential anode materials for lithium ion batteries (LIBs) due to their high theoretical capacities derived from the conversion reaction of MOx + 2xLi + 2xe−![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) M + xLi2O (M = Fe, Co, Ni, etc.).1,2 Fe2O3 is a promising electrode material with the features of high theoretical capacity (1007 mA h g−1), environmental benignity and abundance in nature.3 Despite these distinct advantages, the volume change resulting from the repeated Li+ insertion/extraction seriously destroys the structure of Fe2O3 electrodes, leading to poor cycle stability. Furthermore, the poor conductivity also limits the electrochemical performance. Numerous works have demonstrated that constructing nanostructures with superior structural stability and electrically conductive pathways is an effective strategy to solve these problems.4–6
M + xLi2O (M = Fe, Co, Ni, etc.).1,2 Fe2O3 is a promising electrode material with the features of high theoretical capacity (1007 mA h g−1), environmental benignity and abundance in nature.3 Despite these distinct advantages, the volume change resulting from the repeated Li+ insertion/extraction seriously destroys the structure of Fe2O3 electrodes, leading to poor cycle stability. Furthermore, the poor conductivity also limits the electrochemical performance. Numerous works have demonstrated that constructing nanostructures with superior structural stability and electrically conductive pathways is an effective strategy to solve these problems.4–6
One-dimensional (1D) nanostructures have attracted great attention in energy storage fields because the unique structure can offer fast electron/ion transfer channels and enhance the rate capability. Fe2O3 nanorods,7–9 nanotubes,10,11 nanonoodles12 and nanowires13,14 have been reported to possess satisfied cycle stability and outstanding rate performance as anodes for LIBs. It is found that crossed 1D nanostructures may be more promising candidates for electrode materials because they can offer more rapid transport pathways for ion/electron transport and electrolyte diffusion.15,16 Shen et al. also proposed that “V” type channels can generate an expressway for electrons and ions, which largely contribute to the high capacity and rate performance of electrode materials. The “V” type ZnCo2O4-urchin/carbon cloth electrode delivers a specific capacity of 750 mA h g−1 at a high current density of 18 A g−1.17
Constructing porous structures is believed to be another effective way to design and obtain high-performance electrode materials because they could provide large contact areas for electrode and electrolyte, relieve the structural stress and buffer the volume change during cycling process.18–20 Sun et al. prepared 1D aligned γ-Fe2O3 nanorods anchored on 2D reduced graphene oxide nanosheets and obtained high discharge capacitance of 734 mA h g−1 even at 5C.21 Yu's group reported that the porous α-Fe2O3 nanorods exhibited excellent supercapacitance due to the combined action of 1D nanostructure and the porous nanostructure.22
However, as far as we know there is no literature dealing with the preparation and lithium storage properties of cross-linked porous α-Fe2O3 nanorods. Herein, we develop a facile hydrothermal method to prepare crossed α-Fe2O3 nanorods. Electrochemical measurements demonstrate that the as-prepared α-Fe2O3 nanorods possess high discharge capacity and excellent cycling performance as anode materials for LIBs.
Experimental
Synthesis of cross-linked porous Fe2O3 nanorods
All chemicals were of analytical grade and used without further treatment. The cross-linked porous Fe2O3 nanorods were prepared by a facile hydrothermal method followed by a calcination process. Typically, 1.5 mmol of Fe(NO3)3·9H2O, 0.8 mmol of NH4F and 0.5 mmol of urea were added into 35 mL of deionized water to form a transparent solution. Then the mixture was loaded into a 50 mL Teflon-lined autoclave and heated at 180 °C for 24 h. The FeOOH precursor was obtained after centrifugation and repeatedly washed with anhydrous ethanol and DI water, and dried at 60 °C for 6 h. Finally, after annealing the FeOOH precursor in air at 450 °C for 2 h, cross-linked porous α-Fe2O3 nanorods were obtained. As a comparison, Fe2O3 nanoparticles were prepared by changing the dosage of NH4F into 0.2 mmol.Materials characterization
Powder X-ray diffraction (XRD, Rigaku D/Max-2500, Cu Kα radiation, λ = 0.15418 nm), field emission scanning electron microscopy (FESEM; JEOL-7500, 2 keV) and transmission electron microscope (TEM, JEOL 2011, 200 keV) were employed to investigate the crystal structure and morphologies of the as-prepared samples. The energy dispersive spectroscopy (EDS) elemental maps were also collected. Nitrogen adsorption–desorption isotherms were collected on a Quantachrome Autosorb-IQ2 analyzer. Specific surface areas of the Fe2O3 samples were tested by Brunauer–Emmett–Teller analysis.Electrochemical measurements
Electrochemical performance was tested using CR2032 coin-type cells. The working electrodes were fabricated by mixing the α-Fe2O3 samples (80 wt%), super P (10 wt%) and polytetrafluoroethylene (10 wt%) using EtOH as solvent. Then the slurry was pasted on copper foil and dried at 80 °C for 12 h in vacuum. Metallic lithium was used as cathode. Celgard 2300 film was used as separator and 1 M LiPF6 in ethylene carbonate/dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used as electrolyte. The coin cells were assembled in an argon filled glovebox. Galvanostatic tests were conducted by LAND battery testing system between 3.00 and 0.01 V (vs. Li+/Li). Cyclic voltammetry (CV) measurements were carried out on electrochemical workstation (Zahner IM6e) from 0.01 to 3.00 V at a scan rate of 0.1 mV s−1.
1) was used as electrolyte. The coin cells were assembled in an argon filled glovebox. Galvanostatic tests were conducted by LAND battery testing system between 3.00 and 0.01 V (vs. Li+/Li). Cyclic voltammetry (CV) measurements were carried out on electrochemical workstation (Zahner IM6e) from 0.01 to 3.00 V at a scan rate of 0.1 mV s−1.
Results and discussion
Cross-linked porous α-Fe2O3 nanorods were synthesized via a hydrothermal method to obtain FeOOH precursors, followed by a calcination process to obtain α-Fe2O3 products. Fig. 1a shows that the precursor is orthorhombic phase FeOOH (JCPDS no. 81-462). The overall morphology of FeOOH precursor is cross-linked nanorods with 4–6 branches, which are well dispersed (Fig. 1b). The nanorods are about 500–800 nm in length and 100 nm in width. TEM images (Fig. 1c and d) show that the precursor is of solid structure with smooth surface.XRD pattern shown in Fig. 2 demonstrates that after calcined in air, the phase transferred from FeOOH precursor to α-Fe2O3 (JCPDS no. 1-1053). No impurity peak from other phase is detected and the peaks are of sharp shape, indicating the good crystallinity and high purity of the final product. Fig. 3 shows that the crossed rodlike morphology is well maintained after calcination and the size hardly changes. From the large imagination SEM image (Fig. 3b), many nanopores open to the outer surface can be clearly seen, indicating that the nanorods are of porous structure. TEM images further confirm the porous structure of the as-prepared Fe2O3. Moreover, it is observed that these pores of several nanometers in diameter are inlaid in the nanorods and unconnected to each other, which may be helpful for the nanorods to keep good entirety (Fig. 3d and e). The pores may be resulted from the removal of H2O from the FeOOH precursor during the calcination process. The HRTEM images in Fig. 3f and g both show clear and continuous lattice fringes with a spacing of 0.27 nm, corresponding to the (104) plane of α-Fe2O3. The EDS elemental mapping images (Fig. 3h–j) display the quite uniform distribution of Fe and O and the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio is 2
O ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, further confirming the formation of Fe2O3.
3, further confirming the formation of Fe2O3.
 | ||
| Fig. 3 (a and b) SEM images, (c–e) TEM images, (f and g) HRTEM images and (h–j) element mapping images of the cross-linked porous α-Fe2O3 nanorods. | ||
 | ||
| Fig. 4 SEM images of the FeOOH precursors obtained at different hydrothermal reaction times: (a) 0.5 h; (b) 1 h; (c) 2 h; (d) 3 h; (e) 4 h; (f) 10 h. | ||
To investigate the formation process of the cross-linked nanostructure, time-dependent experiments of the FeOOH precursors were conducted. SEM image shown in Fig. 4a displays that when the reaction time was 0.5 h, the FeOOH precursor is of short rod-like nanostructure about 50 nm in length. As the reaction time was increased to 2 h, the nanorods grow longer and become more regular (Fig. 4c). Interestingly, at the reaction time of 3 h, “V” shaped and “X” shaped FeOOH precursors were formed (Fig. 4d). When the reaction time was 10 h, uniform cross-linked FeOOH nanorods with 4–6 branches were formed and keep almost unchanged at even longer reaction time (Fig. 4f). It is well known that introducing inorganic ions is an effective strategy to control the nanocrystal anisotropic growth because the ions tend to be absorbed at special facets, which might change crystal plane surface energy.23,24 In our experiment, F− plays an important role in the growth of the nanorods and the formation of cross-liked structure. From Fig. 5, it is observed that at low F− concentration, the precursor is mainly composed of nanoparticles. Large scale of uniform FeOOH nanoparticles with the diameter of about 70 nm were obtained when the F− was 0.2 mmol. With the increase of F−, cross-linked nanorods are gradually formed. It can be seen that cross-linked nanorods can be easily synthesized by adjusting the reaction time and F− concentration.
 | ||
| Fig. 5 SEM images of the FeOOH precursors obtained with different dosages of NH4F: (a) no added; (b) 0.2 mmol; (c) 0.4 mmol; (d) 0.6 mmol; (e) 0.8 mmol; (f) 1.0 mmol. | ||
The porous characteristic of the cross-linked porous α-Fe2O3 nanorods were analysed with N2 sorption measurement (Fig. 6a). Fig. 6b exhibits a pore size distribution of less than 10 nm, which agrees well with what can be observed in the TEM images. The porous α-Fe2O3 nanorods display a specific surface area of 48.5 m2 g−1, which is larger than that of the Fe2O3 nanoparticles (31.4 m2 g−1). All in all, the as-prepared α-Fe2O3 is of cross-linked nanorods structure, with 4–6 branches, which may benefit for the electron transport. Moreover, it possesses porous structure and high BET surface areas. Those structural features may help to enhance the electrochemical performance.
 | ||
| Fig. 6 (a) Nitrogen adsorption–desorption isotherm and (b) the corresponding pore size distribution of the cross-linked porous α-Fe2O3 nanorods. | ||
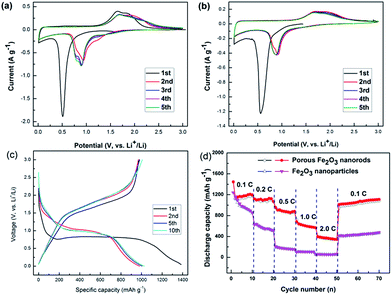
The electrochemical performance of the cross-linked porous Fe2O3 and Fe2O3 nanoparticles electrodes were evaluated by CV and galvanostatic charge–discharge measurements. Fig. 7a shows that during the 1st cathodic polarization process, a sharp peak appears at 0.65 V, which is corresponding to the conversion reaction of Fe(III) to Fe(0) and the formation of a solid electrolyte interphase (SEI) layer.25 In the first anodic polarization process, two peaks located at 1.64 and 1.86 V are detected, owing to the oxidation from metallic Fe(0) to Fe(II) and further transformation to Fe(III).26,27 Both of the full lithiation voltage and dilithiation voltage shift positively in the following cycles, which may be caused by the inherent nanosize effects in the electrode during the following cycles and the improvement of kinetics. Obviously, the area of closed curves of cross-linked porous α-Fe2O3 nanorods electrode is much larger than that of Fe2O3 nanoparticles electrode, indicating higher discharge capacities of cross-linked porous α-Fe2O3 nanorods.
Fig. 7c displays the galvanostatic charge–discharge profiles of cross-linked porous α-Fe2O3 nanorods electrode from 3 to 0.01 V at 0.1C (1C = 1007 mA h g−1). The first discharge curve shows a voltage drop from 3 to 0.75 V, which corresponds to the formation of a Li–Fe–O complex. The obvious potential plateau appearing around 0.75 V is attributed to the transformation of Li–Fe–O to Fe(0) and Li2O. The sloping curve from 0.75 to 0 V is assigned to the formation of a gel-like film and inorganic SEI layer. In the first cycle, the as-prepared cross-linked porous α-Fe2O3 nanorods electrode displays high discharge capacity of 1385.6 mA h g−1, which is much higher than their theoretical capacity. It is mainly due to abundant of electrochemical active sites and grain boundary area of the porous α-Fe2O3 nanorods, as well as the formation of SEI layer. During the following cycles, both of the charging and discharging plateaus move higher, corresponding well to the CV measurements.
The rate performance is a very important measurement of LIBs anodes. The rate capability of the α-Fe2O3 samples is tested by increasing current density from 0.1C, 0.2C, 0.5C, 1.0C, 2.0C and finally back to 0.1C. As shown in Fig. 6d, the cross-linked porous α-Fe2O3 nanorods electrode delivers high reversible capacities of 1183.6, 1095.2, 867.0, 574.2 and 344.2 mA h g−1, correspondingly, which are notably higher than the Fe2O3 nanoparticles electrode. As the current density is back to 0.1C after charge–discharged at 2C, porous α-Fe2O3 nanorods electrode recovers a capacity of 1079.8 mA h g−1, demonstrating superior rate performance and outstanding cycling stability.
To investigate the long cycle durability of the as-obtained α-Fe2O3 electrodes, cycling measurements were conducted at 0.2C for 300 cycles. As shown in Fig. 8a, both α-Fe2O3 electrodes display high initial discharge capacities. However, there is a serious capacity fading in the following 20 cycles, which may be caused by the irreversible reactions and decomposition of electrolyte during the formation and stabilization process of SEI layer.28–31 After that process, the discharge capacity of cross-linked porous α-Fe2O3 nanorods electrode decreases slowly, which still keeps 740.2 mA h g−1 after 300 cycles. Obviously, the porous α-Fe2O3 electrode presents higher discharge capacity and better cycling performance than the Fe2O3 nanoparticles electrode. To further confirm the long cycle life of the cross-linked porous α-Fe2O3 nanorods electrode, cycling measurements were carried out at 1C and 2C. As shown in Fig. 8b, the capacity stabilize at about 600 mA h g−1 at 1C and 520 mA h g−1 at 2C after 300 cycles. The capacity change tendency is in good agreement with that observed at 0.2C.
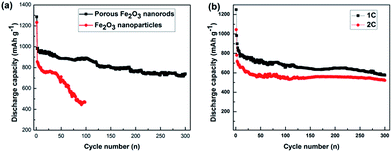 | ||
| Fig. 8 (a) Cycle performance of the as-prepared α-Fe2O3 samples at 0.2C; (b) long cycle stability of the cross-linked porous α-Fe2O3 nanorods electrode at 1C and 2C. | ||
It is believed that the porosity and the durability of nanostructures may contribute to extra-long cycling stability and good rate performance of electrodes. To investigate the effect of the cross-linked structure and inlaid pores of the nanorods on the electrochemical properties, the cycled Fe2O3 electrodes are shown in Fig. 9. According to SEM analysis, the morphology of the cross-linked porous α-Fe2O3 nanorods electrode is well maintained without destruction or pulverization after 50 charge–discharge cycles (Fig. 9a). The pores inlaid in the nanorods can be observed obviously from the TEM image (Fig. 9b). As shown in Fig. 9c, several features may make the as-prepared cross-linked porous α-Fe2O3 nanorods electrode suitable for high-performance lithium ion batteries. First, the 1D nanorod structure and the cross-linked structure shorten the electron transfer pathway effectively. Second, the porous structure of the α-Fe2O3 nanorods increases the electrode–electrolyte contact area and supplies more electroactive sites, as well as facilities the ions transportation, ensuring high electrochemical utilization at high current densities. Third, the pores inlaid in the bulk phase of the α-Fe2O3 nanorods supply inner cavity for the expanding of electrode materials, relieving the structure collapse during the cycling process, which is similar to the effect of mini-hollow structure on energy storage performance.32 All these factors do favour for the high discharge capacity, good rate capability and excellent cycling stability of the as-prepared cross-linked porous α-Fe2O3 nanorods electrode.
Conclusions
In summary, we have reported a facile hydrothermal-calcination method for the synthesis of α-Fe2O3 nanorods with cross-linked structure and inner laid pores. As anode materials for LIBs, the as-prepared α-Fe2O3 sample displays excellent electrochemical performance. The 1D nanostructure and the cross-linked structure facilitate the rapid transport of electrons in the anode materials, which may do favor to the rate capabilities. Furthermore, the pores distributed in the bulk phase of the material can enlarge electrode–electrolyte contact area and supply space for the volume expanding, enables such electrode material to possess high discharge capacity and good cycle life. These results suggest that such cross-linked porous α-Fe2O3 nanorods are very promising for high-performance lithium ion batteries.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21376113, 51401094), the Natural Science Foundation of Jiangsu Province (BK20160213) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- E. Kang, Y. S. Jung, A. S. Cavanagh, G.-H. Kim, S. M. George, A. C. Dillon, J. K. Kim and J. Lee, Adv. Funct. Mater., 2011, 21, 2430–2438 CAS.
- Z. Wang, L. Zhou and X. W. Lou, Adv. Mater., 2012, 24, 1903–1911 CrossRef CAS PubMed.
- B. Wang, J. S. Chen, H. B. Wu, Z. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146–17148 CAS.
- K. Cao, L. Jiao, H. Liu, Y. Liu, Y. Wang, Z. Guo and H. Yuan, Adv. Energy Mater., 2015, 5, 1401421 Search PubMed.
- J. Zhu, Z. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Yan, Energy Environ. Sci., 2013, 6, 987–993 CAS.
- K. S. Lee, S. Park, W. Lee and Y. S. Yoon, ACS Appl. Mater. Interfaces, 2016, 8, 2027–2034 CAS.
- C. T. Cherian, J. Sundaramurthy, M. Kalaivani, P. Ragupathy, P. S. Kumar, V. Thavasi, M. V. Reddy, C. H. Sow, S. G. Mhaisalkar, S. Ramakrishna and B. V. R. Chowdari, J. Mater. Chem., 2012, 22, 12198–12204 RSC.
- S. Chen, Y. Xin, Y. Zhou, F. Zhang, Y. Ma, H. Zhou and L. Qi, J. Mater. Chem. A, 2015, 3, 13377–13383 CAS.
- Y. M. Lin, P. R. Abel, A. Heller and C. B. Mullins, J. Phys. Chem. Lett., 2011, 2, 2885–2891 CrossRef CAS.
- Z. Wang, D. Luan, S. Madhavi, C. M. Li and X. W. Lou, Chem. Commun., 2011, 47, 8061–8063 RSC.
- J. Chen, L. Xu, W. Li and X. Guo, Adv. Mater., 2005, 17, 582–586 CrossRef CAS.
- Q. Q. Xiong, J. P. Tu, X. Ge, X. L. Wang and C. D. Gu, J. Power Sources, 2015, 274, 1–7 CrossRef CAS.
- B. Liu, X. Wang, B. Liu, Q. Wang, D. Tan, W. Song, X. Hou, D. Chen and G. Shen, Nano Res., 2013, 6, 525–534 CrossRef CAS.
- L. Liao, Z. Zheng, B. Yan, J. X. Zhang, H. Gong, J. C. Li, C. Liu, Z. X. Shen and T. Yu, J. Phys. Chem. C, 2008, 112, 10784–10788 CAS.
- Y. Zhong, M. Yang, X. Zhou and Z. Zhou, Mater. Horiz., 2015, 2, 553–566 RSC.
- W. Zhou, L. Lin, W. Wang, L. Zhang, Q. Wu, J. Li and L. Guo, J. Phys. Chem. C, 2011, 115, 7126–7133 CAS.
- B. Liu, X. Wang, B. Liu, Q. Wang, D. Tan, W. Song, X. Hou, D. Chen and G. Shen, Nano Res., 2013, 6, 525–534 CrossRef CAS.
- M. Chen, J. Liu, D. Chao, J. Wang, J. Yin, J. Lin, H. Jin Fan and Z. X. Shen, Nano Energy, 2014, 9, 364–372 CrossRef CAS.
- J. Chen, T. Zhu, X. Yang, H. Yang and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 13162–13164 CrossRef CAS PubMed.
- L. Zhang, H. B. Wu, S. Madhavi, H. H. Hng and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 17388–17391 CrossRef CAS PubMed.
- X. Wang, J. Mujtaba, F. Fang, M. Ahmad, H. Arandiyan, H. Yang, G. Sun and H. Sun, RSC Adv., 2015, 5, 91574–91580 RSC.
- S. Chaudhari, D. Bhattacharjya and J. S. Yu, RSC Adv., 2013, 3, 25120–25128 RSC.
- Z. F. Dou, C. Y. Cao, Q. Wang, J. Qu, Y. Yu and W. G. Song, ACS Appl. Mater. Interfaces, 2012, 4, 5698–5703 CAS.
- M. Cao, T. Liu, S. Gao, G. Sun, X. Wu, C. Hu and Z. Wang, Angew. Chem., Int. Ed., 2005, 44, 4197–4201 CrossRef CAS PubMed.
- Y. Huang, Z. Lin, M. Zheng, T. Wang, J. Yang, F. Yuan, X. Lu, L. Liu and D. Sun, J. Power Sources, 2016, 307, 649–656 CrossRef CAS.
- M. Srinivasan and S. Chaudhari, J. Mater. Chem., 2012, 22, 23049–23056 RSC.
- J. Kan and Y. Wang, Sci. Rep., 2013, 3, 3502 Search PubMed.
- S. K. Behera, Chem. Commun., 2011, 47, 10371–13980 RSC.
- M. F. Hassan, M. M. Rahman, Z. P. Guo, Z. X. Chen and H. K. Liu, Electrochim. Acta, 2010, 55, 5006–5013 CrossRef CAS.
- J. Zhang, T. Huang and Z. Liu, Electrochem. Commun., 2013, 29, 17–20 CrossRef CAS.
- X. Huang, H. Yu, J. Chen, Z. Lu, R. Yazami and H. H. Hng, Adv. Mater., 2014, 26, 1296–1303 CrossRef CAS PubMed.
- K. Cao, L. Jiao, H. Xu, H. Liu, H. Kang, Y. Zhao, Y. Liu, Y. Wang and H. Yuan, Adv. Sci., 2016, 3, 2991–2997 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |