A new class of electropolymerized conducting film from the pyrimidine family for the simultaneous determination of ascorbic acid and dopamine
Ayyadurai Kannana,
Arumugam Sivanesanb,
Govindasamy Kalaivania and
Ranganathan Sevvel*a
aPost Graduate and Research Department of Chemistry, Vivekananda College, Tiruvedakam West, Madurai-625 234, Tamil Nadu, India. E-mail: sevvelr@gmail.com; Fax: +91 4543 258358; Tel: +91 98657 08536
bNanotechnology and Molecular Sciences Discipline, Faculty of Science and Engineering, Queensland University of Technology, 2 George St. Gardens Point Campus, Brisbane 4001, QLD, Australia
First published on 28th September 2016
Abstract
In this study, we report the electropolymerization of 4-amino-6-hydroxy-2-mercaptopyrimidine (AHMP) in 0.1 M H2SO4 on a glassy carbon electrode (GCE) and its application for simultaneous as well as the selective determination of ascorbic acid (AA) and dopamine (DA) at pH 4. The electropolymerized AHMP (p-AHMP) film was characterized by scanning electron microscopy (SEM), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The p-AHMP film showed a redox couple in 0.1 M H2SO4 and a p-AHMP film deposited by 10 cycles showed high sensitivity than that with 5, 15 and 20 deposited cycles. The surface coverage of the p-AHMP film was found to be 5.24 × 10−10 mol cm−2, suggesting the formation of a nanostructured electroactive film on the GCE. Electrochemical impedance spectroscopy study revealed that the modified electrode underwent a facile electron transfer compared to the bare GCE. CV and differential pulse voltammetry (DPV) were employed to demonstrate the electrochemical behaviour of p-AHMP/GCE, which exhibited good electrocatalytic performance towards AA and DA without any electron mediator. The detection limit of AA and DA were found to be 1.20 and 0.28 μM, respectively (S/N = 3) by the DPV method. The modified electrode was successfully utilized to determine AA and DA in real samples such as dopamine injection and vitamin C tablets.
1. Introduction
The last decade has witnessed a tremendous volume of publications devoted to conductive polymers, and this field continues to attract the interest of numerous academic and industrial researchers due to its diverse electronic conducting, optical, physical, chemical and biochemical properties.1–6 These distinctive properties of conducting polymers have been constantly finding applications in photovoltaic devices,7 electrochromic displays,8 energy and charge storage devices,9 light emitting diodes,10 electrocatalysis,11 and chemical and biological sensors.2 It is well documented that conducting polymers prepared via the electropolymerization method possess high stability, uniformity and reproducibility.12 Electropolymerization is a promising technique to immobilize the polymer on GCE. Moreover, the advantages of electropolymerized electrodes are precise control in thickness of the film, a wide choice of electrode materials, strong adherence power on the electrode surface, broad potential window, large surface area which promotes higher turn-over efficiency, ease of preparation and sensitivity.13,14 Typical examples of electrochemically prepared conducting polymers are polypyrrole, polythiophene, polyaniline and their derivatives.14–16 Recently, an electropolymerized conducting film prepared from substituted thiadiazole compounds has been widely used for electrochemical sensor applications.17,18 Thus, despite the numerous conducting polymer films available, researchers are continuously exploring varieties of compounds to discover a new conducting polymer. To the best of our knowledge, we are the first to use a pyrimidine type compound to prepare conducting polymer via the electropolymerization method.Dopamine (DA) and ascorbic acid (AA) usually coexist in our body fluids, and both play an important role in human metabolism.19 DA is an electroactive catecholamine group of neurohormone produced in several areas of the brain, including the substantianigra and the ventral tegmental area, and is released by the hypothalamus.20 Deficiency of DA in the central nervous system causes several neurological diseases, which includes Parkinson's disease,21 schizophrenia22 and attention deficit hyperactivity disorder.23 Therefore, it is important to develop a simple and rapid method for determining DA in body fluids. Similarly, AA being an antioxidant and vital vitamin also plays several important roles in our body, which includes prevention of scurvy, mental illness, cancer and fertility related issues.24 Most of the time both DA and AA coexist in body fluids, and therefore it is essential to determine them either selectively in the presence of the other, or simultaneously. Since bare electrodes failed to separate the signals, modified electrodes were used for the determination of DA and AA, and there are plenty of literature reports discussing the electrochemical way of determining the above two compounds.25–27 Despite this huge body of literature, our aim was to test the potential of the present pyrimidine type of conducting polymer as an electrochemical sensor to the well-known system of AA and DA.
The present work is about the preparation of 4-amino-6-hydroxy-2-mercaptopyrimidine conducting polymer on a glassy carbon electrode via an electropolymerization method, characterization of the film by electrochemical and SEM methods and subsequent sensor application for the simultaneous determination of AA and DA.
2. Experimental
2.1. Chemicals and materials
The 4-amino-6-hydroxy-2-mercaptopyrimidine monohydrate (AHMP), ascorbic acid (AA) and dopamine (DA) were purchased from Sigma Aldrich and were used as received. All the other chemicals were of analytical grade and used directly without further purification. Diverse pH values of phosphate buffer (PB) solutions were prepared using various ratios of 0.1 M Na2HPO4 and NaH2PO4. A dilute solution (0.1 M) of H3PO4 was used for preparing PB solution with lower pH values. Double distilled water was used to prepare all the experimental solutions. All of the electrochemical experiments were carried out in an inert atmosphere.Polishing slurries (0.5 μm and 0.3 μm) and pads (Microcloth®) were purchased from Buehler, Germany. Glassy carbon (GC) working electrodes with a 3 mm diameter and platinum counter electrodes were purchased from CH Instruments (Austin, TX, USA). A dry leak less electrode (DRIREF-2) was purchased from World Precision Instruments, USA and used as a reference electrode. Glassy carbon discs with a 1 cm diameter were purchased from HTW, Germany and were used as SEM substrates.
2.2. Instrumentation
All electrochemical experiments were carried out in a CHI 6088D (Austin, TX, USA) electrochemical workstation with a custom-made three-electrode cell setup. SEM measurements were performed using a FEI Nova™ NanoSEM Scanning Electron Microscope 450 with an accelerating voltage of 10 kV under high vacuum.2.3. Fabrication of AHMP conducting polymer on a GC electrode
The GC working electrode was mirror polished with 0.5 μm and 0.3 μm alumina slurries, respectively, and subsequently sonicated in double distilled water for 10 min to get rid of the physically adsorbed alumina particles from the GC surface. The quality of the polished electrode was electrochemically tested using a [Fe(CN)6]3−/4− redox couple in 0.1 M KCl. Electropolymerization of AHMP on a GC electrode was carried out by 10 successive potential sweeps between −0.5 V and 2 V at a 0.05 V s−1 scan rate in 0.1 M H2SO4 solution containing 1 mM AHMP. After electropolymerization, the AHMP polymerized GC electrode (p-AHMP/GC) was removed from the solution and washed with ample amount of 0.1 M H2SO4 to remove the free monomer molecules from the electrode surface. The p-AHMP/GC electrode was then subsequently washed with double distilled water and used for further studies.3. Results and discussion
3.1. Electropolymerization of AHMP on GC electrode
Fig. 1 displays the 10 continuous cyclic voltammograms (CVs) corresponding to electropolymerization of 0.1 M AHMP at a GC electrode over a −0.5 to 2 V potential range in 0.1 M H2SO4 solution. It can be seen from Fig. 1 that oxidation and reduction peaks were observed, which indicates that the oxidation of AHMP monomer to form radical cation and the reduction wave were due to the reduction of the dimer and/or oligomer generated by the polymerization of AHMP radical cations.28,29 | ||
| Fig. 1 Cyclic voltammograms (10 successive cycles) obtained for 1 mM AHMP in 0.1 M H2SO4 on GCE at a 50 mV s−1 scan rate. | ||
During electropolymerization, it should be noted that the peak current increased concurrently from the first cycle to the tenth indicating a continuous increase of surface coverage of the p-AHMP conducting film on GCE.30 Upon further oxidation, the polymerization of AHMP would be diminished by the previously formed p-AHMP on a GC surface due to a decrease of the electron transfer rate.31 To further polymerize unreacted monomer on the inside of the p-AHMP/GC electrode, potential scanning was performed between the −0.5 and 2 V potential window in AHMP monomer free 0.1 M H2SO4 at a 50 mV s−1 scan rate.
3.2. Electrochemical behavior of p-AHMP film
The cyclic voltammograms (CVs) of the p-AHMP film deposited on the glassy carbon electrode surface by 5, 10, 15 and 20 potential sweeps in monomer free 0.1 M H2SO4 at a 50 mV s−1 scan rate are shown in Fig. 2A. It can be seen from Fig. 2A, that the oxidation and reduction peak currents were increased gradually with continuous polymerization by potential scanning from 5 to 20 cycles. Moreover, increased peak separation was also observed. Peak separations of 87, 90, 110 and 180 mV were observed for the p-AHMP films deposited by 5, 10, 15 and 20 cycles respectively. This indicates that the electron transfer reaction of the p-AHMP film diminished when increasing the deposition cycles.32 This redox reaction observed in this region is due to a proton and electron addition and elimination reaction at the –NH– sites in the p-AHMP film on GCE.31,32 The effect of potential scan rate (υ) on the electrochemical properties of p-AHMP modified GCE was studied by CV in AHMP free 0.1 M H2SO4 solution at different scan rates (Fig. 2B).When the scan rate was increased from 20 to 1000 mV s−1, both the oxidation and reduction peak currents linearly increased without any change in redox potential, which confirmed that the electrode reaction was a surface confined redox process and reversible redox reaction. Plots of anodic and cathodic peak currents were linearly dependent on υ in the 20 to 1000 mV s−1 range (inset). The surface coverage was evaluated using the equation Ip = n2F2υAГ/4RT or Г = Q/nFA, where Q is the charge obtained by integrating the anodic peak current under the background correction, n is the number of electrons involved in the electron transfer process, F is the Faraday constant and A is the geometric area of the GC electrode (0.0707 cm2).33,34 The surface coverage of 5.24 × 10−10 mol cm−2 was obtained for p-AHMP film deposited by 10 cycles.
3.3. Effect of electropolymerization cycles
The effect of the electropolymerization cycles of AHMP on the oxidation peak currents responses of 10 μM of DA at p-AHMP was investigated by DPV in Fig. 3. As can be seen in this figure, the p-AHMP film deposited by 10 cycles showed a maximum peak current compared to other 5, 15, and 20 deposited cycles. The lower peak current of the polymer film observed in 5 deposited cycles indicated insufficient polymer film growth on the GC surface. The similar effect was also observed in 15 and 20 deposited cycles suggesting that the polymer film inhibited the electron transfer rate due to the formation of a thick film on the GC surface. Therefore, the polymer film used in this study was prepared by 10 continuous potential cycles. | ||
| Fig. 3 Effect of electropolymerization cycles of AHMP on the oxidation current responses of 10 μM of DA at p-AHMP/GCE in 0.1 M PBS. | ||
3.4. Electrochemical impedance spectroscopy study
Electrochemical impedance spectroscopy (EIS) is an effective tool for studying resistivity changes of the electrode surface during the modification process. EIS contains both a semicircle part at high frequency and a linear part at low frequency, which correspond to an electron transfer limited process and diffusion process, respectively.35 The diameter of the semicircle in the impedance spectrum is equal to the charge transfer resistance, Rct. Fig. 4 shows the results of impedance spectra of a bare GCE (curve a) and p-AHMP/GCE (curve b). On the bare GCE, the value of Rct was obtained to be 6954 Ω (curve a). After fabrication of p-AHMP on the GCE surface, the value of Rct was obtained to be 2541 Ω (curve b). The decreased Rct value for p-AHMP/GCE showed that the conductive p-AHMP film was attached to the GCE surface and this indicates that the electron transfer process of the modified electrode was relatively fast compared to bare GCE. | ||
Fig. 4 Nyquist plots showing faradaic impedance measurements of bare GCE (a), p-AHMP/GCE (b) in 1 mmol L−1 K3[Fe(CN)6]/K4[Fe(CN)6] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 0.1 M KCl. 1) containing 0.1 M KCl. | ||
3.5. SEM of modified electrode
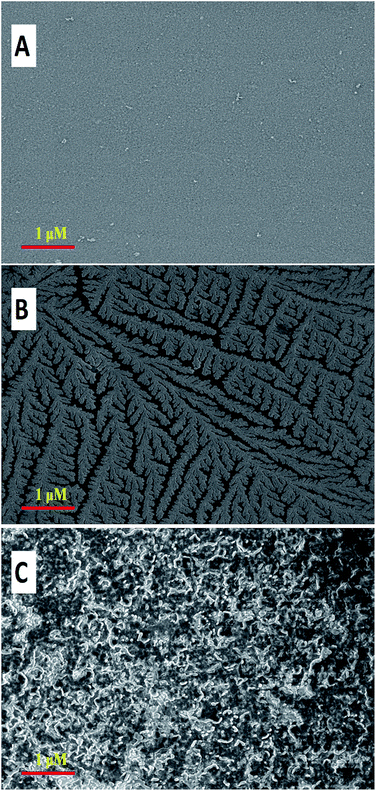
The surface morphology of the modified film was investigated by SEM. Fig. 5A–C illustrate the SEM images of the p-AHMP film deposited by 5, 10 and 15 cycles, respectively. In Fig. 5A, a surface with a slight roughness is observed. As shown in Fig. 5B, a branch-like structure with jagged leaves of p-AHMP film was observed on the electrode surface. After increasing of deposition cycles the dendrites' structure changed into a homogeneous and rough surface structure (Fig. 5C).3.6. The effect of pH on the oxidation of AA and DA in the mixture
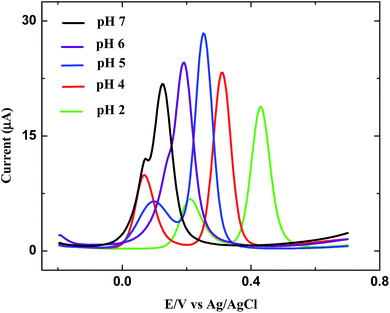
The influence of pH value on the simultaneous determination of AA and DA at p-AHMP/GCE was investigated by DPV. From Fig. 6, we understand that the pH value played a crucial role. At and above pH 5, there was no marked separation of peaks of AA and DA but below this value, a clear, distinct peak for AA and DA was observed. AA and DA gave the highest current response and less positive potential oxidation at pH 4 compared with pH 5 and pH 2. The reason may be related to the effective electrostatic attraction between anionic and cationic species of analytes and the p-AHMP modified GC electrode at pH 4. Therefore we chose pH 4 PB solution for the determination of AA and DA.363.7. Electrochemical behaviour of AA and DA in a mixture at a bare and p-AHMP modified GC electrode
Fig. 7 displays the cyclic voltammograms obtained for 0.2 mM of each AA and DA in a mixture at a bare and p-AHMP modified GC electrode in 0.1 M PBS (pH 4). The bare GC electrode shows the undefined oxidation peaks for AA and DA, at 0.285 V and 0.440 V, respectively, in the first scan (curve a). In the subsequent scans, the oxidation peaks of AA and DA were shifted to slightly positive potential with a notable decrease in the oxidation current (curve b). This result suggested that the bare GC electrode is not optimal for stable determination of AA and DA. Unlike the bare GC electrode, the p-AHMP modified electrode showed well-defined and distinguished oxidation peaks for AA and DA at 0.130 V and 0.360 V, respectively; with a two fold excess of higher oxidation peak currents (curve c), the peaks were shifted to a less positive potential compared to the bare GC electrode. The separation between the two peak potentials was 0.230 V, which is more suitable for the selective and simultaneous determination of AA and DA in a mixture. On the subsequent scans (curve d), the oxidation peak potentials of AA and DA at the p-AHMP modified electrode were stable (10th scan) (curve d). The large peak separation of AA and DA, greatly increased the peak currents and stable cyclic voltammograms permitted us to simultaneously determine these species with high sensitivity.3.8. Simultaneous determination of AA and DA
The main objective of this study was to determine both AA and DA simultaneously. The utilization of the p-AHMP modified GC electrode for the simultaneous determination of AA and DA was demonstrated by simultaneously changing the concentration of AA and DA. Based on the CV studies, the order of sensitivity of these analytes at the p-AHMP electrode was DA > AA. Therefore, we chose 20 μM AA and 5 μM DA as increments for the differential pulse voltammetric measurements. The DP voltammetric results showed the simultaneous determination of AA and DA with two well-defined and distinguished anodic peak potentials of 75 mV and 305 mV, which correspond to the oxidation of AA and DA (curve a) in 0.1 M PBS (pH 4) at a p-AHMP modified electrode, as shown in Fig. 8. No shift in the oxidation peak potentials of AA and DA were observed on further addition of the respective analyte to 0.1 M PBS (curve b–l). The peak currents of AA and DA increased linearly with 0.996 and 0.999 correlation coefficients, respectively, when increasing their concentration from 20 to 400 μM for AA and 5 to 100 μM for DA.3.9. Selective determination of AA and DA
The other objective of this study was the selective determination of DA and AA at p-AHMP/GCE in a mixture. It was investigated by keeping the concentration of one species constant and changing the concentration of other species. Fig. 9A shows DPVs obtained for the incremental addition of 5 μM DA in the presence of 1 mM of AA in 0.1 M PB solution. A clear voltammogram was obtained for 5 μM DA in the presence of a 200 fold excess of AA, suggesting that the determination of very low DA concentration is possible even in the presence of a high concentration of AA. With the incremental addition of 5 μM DA to 1 mM AA, the oxidation peak current of DA had linearly increased with a 0.9996 correlation coefficient. No shift of anodic peak potentials of AA and DA were found.Similarly, Fig. 9B shows the DPVs obtained for the increment of 20 μM AA in the presence of 0.3 mM DA. A clear voltammetric signal was observed for AA even in the presence of a higher DA concentration. The oxidation peak current of AA linearly increased with a 0.9994 correlation coefficient without a change in the anodic peak potentials of AA and DA. These results suggest that the p-AHMP modified electrode can be used for selective determination of DA and AA. The detection limits of AA and DA are found to be 1.20 and 0.28 μM (S/N = 3), respectively. The performance of the fabricated AA and DA sensors are very much comparable to literature values (Table 1).37–43 It is evident from Table 1 that the newly designed p-AHMP modified electrode exhibited a relatively low detection limit, high sensitivity and wide linear range.
| Electrodes | Linear range (μM) | Detection limit (μM) | References | ||
|---|---|---|---|---|---|
| AA | DA | AA | DA | ||
| Poly-ATD/GCE | 30–300 | 5–50 | 2.01 | 0.33 | 37 |
| GS-PTCA | 20–420 | 0.4–370 | 5.60 | 0.13 | 38 |
| Poly(evans blue)/GCE | 5–105 | 1–10 | 0.3 | 0.25 | 39 |
| P-4-ABA/GCE | 20–800 | 5–100 | 5.0 | 1.0 | 40 |
| Poly(acid chrome blue K)/GCE | 50–1000 | 1–200 | 10.0 | 0.5 | 41 |
| Fe3O4/rGO/GC | 160–722 | 0.4–3.5 | 20 | 0.08 | 42 |
| CTAB-GO/MWNT/GCE | 5–300 | 5–500 | 1.0 | 1.5 | 43 |
| p-AHMP/GCE | 20–350 | 5–115 | 1.20 | 0.28 | This work |
3.10. Anti-interference ability
In order to evaluate the anti-interference ability of the p-AHMP film, some common physiological interferents such as Na+, K+, NH4+, Mg2+, Ca2+, Cl−, F−, CO32−, SO42−, citric acid, lysine, glycine and cysteine were employed towards the selective detection of AA and DA. No change in current response was observed for 50 μM of AA and DA in the presence of 1000 fold excess of these interferents. This indicated that the p-AHMP modified GC electrode was highly selective towards the AA and DA.3.11. Stability and reproducibility
It is important that modified electrodes should possess reproducibility and stability for longer periods. The reproducibility of the p-AHMP modified GC electrode was estimated from the response to the same sample at five modified electrodes prepared under the same conditions. The reproducibility of these modified electrodes had a relative standard deviation of 3.10%. The operational reproducibility of the sensor was measured by modified GC electrodes by repetitive detection of 200 μM AA and DA in a mixture. The results of 15 successive measurements showed relative standard deviations in peak currents of 2.5% and 1.9% for AA and DA, respectively. The modified electrode showed good reproducibility. Also the stability of the modified electrode was investigated over a period of one month by storing it in 0.1 M PBS pH 4 at 4 °C. The current response of AA had decreased by 2.9% in the first 15 days and 3.8% in the next 15 days and DA was 2.5% in first 15 days and 3.1% in the next 15 days, which indicated that the p-AHMP modified GC electrode exhibited good stability. Therefore, the p-AHMP modified GC electrode could be surely applied to detect AA and DA concentration in practical clinical analysis.3.12. Detection of AA and DA in a real sample
In order to validate the practical applicability of the modified electrode for real samples, the AA content in vitamin C tablets and DA in dopamine hydrochloride drug injection were suitably diluted with 0.1 M PBS (pH 4). The real samples were carefully analyzed using a calibration plot. A known concentration solution of AA and DA were added to the real sample to study the recovery. The results are summarized in Table 2, which indicate that the recoveries of the spiked samples were in the range of 97.8–104.4%. These results suggest that the p-AHMP/GC electrode is reliable for detecting AA and DA in tablets and injection, respectively.| Sample | Content (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%) (n = 5) |
|---|---|---|---|---|---|
| Determination of DA in dopamine hydrochloride injection | |||||
| 1 | 20 | 20 | 39.1 | 97.8 | 3.55 |
| 2 | 40 | 20 | 62.5 | 104.1 | 1.80 |
| 3 | 60 | 20 | 78.6 | 98.3 | 2.50 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Determination of AA in vitamin C tablets | |||||
| 1 | 30 | 30 | 60.5 | 100.8 | 2.44 |
| 2 | 50 | 30 | 83.5 | 104.4 | 3.53 |
| 3 | 80 | 30 | 118.3 | 98.6 | 4.06 |
4. Conclusion
A novel p-AHMP modified glassy carbon electrode was fabricated. The p-AHMP film was characterized by CV, SEM and EIS. The p-AHMP film deposited by 10 cycles showed good electrocatalytic activity towards AA and DA. The modified electrode was used to determine AA and DA simultaneously and selectively in the presence of a higher concentration of the other. Under the optimal experimental condition, the anodic peak currents of AA and DA increased linearly within 20–350 μM and 5–115 μM concentration ranges with correlation coefficients of 0.9994 and 0.9996, respectively. The detection limits were 1.20 and 0.28 μM for AA and DA, respectively (S/N = 3). The p-AHMP/GCE was employed to determine the dopamine in injection and ascorbic acid in vitamin C tablets and satisfying results were achieved. The successful application of this modified electrode indicated that the p-AHMP film provides a new way for designing a selective and simultaneous sensor for AA and DA.Acknowledgements
Financial support from the University Grants Commission – South Eastern Regional Office (UGC – SERO), Hyderabad, for the award of Minor Research Project (No. F MRP-5855/15) (SERO-UGC) is gratefully acknowledged by AK. AK, GK and RS would like to thank The Management and The Principal, Vivekananda College, Tiruvedakam West for their support and encouragement. The authors thank Dr P. Veluchamy, Senior Consultant Engineer, First Solar, USA for donating CHI Electrochemical workstation to Vivekananda College.References
- C. Li, H. Bai and G. Shi, Chem. Soc. Rev., 2009, 38, 2397–2409 RSC.
- M. Gerard, A. Chaubey and B. D. Malhotra, Biosens. Bioelectron., 2002, 17, 345–434 CrossRef CAS PubMed.
- H. Yoon and J. Jang, Adv. Funct. Mater., 2009, 19, 1567–1576 CrossRef CAS.
- J. Janata and M. Joscowicz, Nat. Mater., 2003, 2, 19–24 CrossRef CAS PubMed.
- D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537–2574 CrossRef CAS PubMed.
- M. Ates, Mater. Sci. Eng., C, 2013, 33, 1853–1859 CrossRef CAS PubMed.
- K. M. Coakley and M. D. McGehee, Chem. Mater., 2004, 16, 4533–4542 CrossRef CAS.
- G. Nie, L. Zhou, Q. Guo and S. Zhang, Electrochem. Commun., 2010, 12, 160–163 CrossRef CAS.
- S. Ghosh, T. Maiyalagan and R. N. Basu, Nanoscale, 2016, 8, 6921–6947 RSC.
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Bredas, M. Logdlund and W. R. Salaneck, Nature, 1999, 397, 121–128 CrossRef CAS.
- V. M. Vasantha and S.-M. Chen, J. Electroanal. Chem., 2006, 592, 77–87 CrossRef CAS.
- X. Lu, W. Zhang, C. Wang, T.-C. Wen and Y. Wei, Prog. Polym. Sci., 2011, 36, 671–712 CrossRef CAS.
- S.-M. Park, Electrochemistry of π conjugated polymers, in Handbook of Organic Conductive Molecules and Polymers, ed. H. S. Nalwa, Chichester, Wiley, 3rd edn, 1997, p. 429 Search PubMed.
- A. F. Diaz, K. K. Kanazawa and G. P. Gardini, J. Chem. Soc., Chem. Commun., 1979, 14, 635–636 RSC.
- G. Tourillon and F. Garnier, J. Electroanal. Chem., 1982, 135, 173–178 CrossRef CAS.
- E. M. Genies and M. Lapkowski, J. Electroanal. Chem., 1987, 236, 173189–173197 Search PubMed.
- S. B. Revin and S. A. John, Electrochim. Acta, 2011, 56, 8934–8940 CrossRef CAS.
- L. Zhang, D. Yang and L. Wang, Electrochim. Acta, 2013, 111, 9–17 CrossRef CAS.
- X. Zheng, X. Zhou, X. Ji, R. Lin and W. Lin, Sens. Actuators, B, 2013, 178, 359–365 CrossRef CAS.
- K. Jackowska and P. Krysinski, Anal. Bioanal. Chem., 2013, 405, 3753–3771 CrossRef CAS PubMed.
- J. H. Kim, J. M. Auerbach, J. A. R. Gomez, I. Velasco, D. Gavin, N. Lumelsky, S. H. Lee, J. Nguyen, R. S. Pernaute, K. Bankiewicz and R. Mckay, Nature, 2002, 418, 50–56 CrossRef CAS PubMed.
- R. M. Wightman, L. J. May and A. C. Michael, Anal. Chem., 1998, 60, 769A–779A CrossRef.
- J. W. Mo and B. Ogorevc, Anal. Chem., 2001, 73, 1196–1202 CrossRef CAS PubMed.
- N. F. Atta, M. F. El-Kady and G. Ahmed, Anal. Biochem., 2010, 400, 78–88 CrossRef CAS PubMed.
- K. Jackowska and P. Krysinski, Anal. Bioanal. Chem., 2013, 405, 3753–3771 CrossRef CAS PubMed.
- A. M. Pisoschi, A. Pop, A. I. Serban and C. Fafaneata, Electrochim. Acta, 2014, 121, 443–460 CrossRef CAS.
- M. Sajid, M. K. Nazal, M. Mansha, A. Alsharaa, S. M. S. Jillani and C. Basheer, Trends Anal. Chem., 2016, 76, 15–29 CrossRef CAS.
- D. Lan and L. Zhang, J. Electroanal. Chem., 2015, 757, 107–115 CrossRef CAS.
- P. Kalimuthu and S. A. John, Electrochem. Commun., 2009, 11, 367–370 CrossRef CAS.
- P. Kalimuthu and S. A. John, Electrochim. Acta, 2009, 55, 183–189 CrossRef CAS.
- L. Zhang and L. Wang, J. Solid State Electrochem., 2013, 17, 691–700 CrossRef CAS.
- S. B. Revin and S. A. John, Electrochim. Acta, 2011, 56, 8934–8940 CrossRef CAS.
- E. Laviron, J. Electroanal. Chem., 1979, 101, 19–28 CrossRef CAS.
- S. Thiagarajan, R.-F. Yang and S.-M. Chen, Bioelectrochemistry, 2009, 75, 163–169 CrossRef CAS PubMed.
- Y. Oztekin, M. Tok, E. Bilici, L. Mikoliunaite, Z. Yazicigil, A. Ramanaviciene and A. Ramanavicius, Electrochim. Acta, 2012, 76, 201–207 CrossRef CAS.
- C. Wang, R. Yuan, Y. Chai, S. Chen, F. Hu and M. Zhang, Anal. Chim. Acta, 2012, 741, 15–20 CrossRef CAS PubMed.
- P. Kalimuthu and S. A. John, Talanta, 2010, 80, 1686–1691 CrossRef CAS PubMed.
- W. Zhang, Y. Q. Chai and R. Yuan, Anal. Chim. Acta, 2012, 756, 7–12 CrossRef CAS PubMed.
- L. Lin, J. Chen, H. Yao, Y. Chen, Y. Zheng and X. Lin, Bioelectrochemistry, 2008, 73, 11–17 CrossRef CAS PubMed.
- X. Zheng, X. Zhou, X. Ji, R. Lin and W. Lin, Sens. Actuators, B, 2013, 178, 359–365 CrossRef CAS.
- R. Zhang, G. D. Jin and X. Y. Hu, Sens. Actuators, B., 2009, 138, 174–181 CrossRef CAS.
- H. Teymourian, A. Salimi and S. Khezrian, Biosens. Bioelectron., 2013, 49, 1–8 CrossRef CAS PubMed.
- Y. J. Yang and W. Li, Biosens. Bioelectron., 2014, 56, 300–306 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |