Large areal mass, flexible and freestanding polyaniline/bacterial cellulose/graphene film for high-performance supercapacitors
Rong Liu,
Lina Ma,
Shu Huang,
Jia Mei,
Jun Xu and
Guohui Yuan*
School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, 150001, P. R. China. E-mail: yghhit@163.com
First published on 7th November 2016
Abstract
Flexible supercapacitors are promising energy storage devices for wearable and portable electronics. The key challenge for practical applications is to exploit a simple, scalable and low-cost strategy for fabricating large areal mass, high areal performance and mechanically tough flexible electrodes. Here, we describe flexible and freestanding electrodes with high mass loading in the range of 7–13 mg cm−2 made of polyaniline (PANI)/bacterial cellulose (BC)/graphene (GN) conductive paper through a simple filtering method. This hierarchically flexible electrode exhibits an ultrahigh areal capacitance of 6.15 F cm−2 at a large mass of 12.9 mg cm−2, which is about two to five times higher than that of most polymer flexible electrodes. A symmetric supercapacitor using this flexible electrode is able to offer high areal capacitance (1.93 F cm−2) and energy density (0.17 mW h cm−2). Therefore, this flexible and freestanding paper electrode holds great promise for flexible energy storage devices.
1. Introduction
Supercapacitors feature higher charging/discharging rate capability, higher power density, longer life-cycle and safer operation than batteries and superior energy density to conventional physical capacitors, and therefore are widely used as energy storage devices.1–7 Generally, supercapacitors can be classified into electrical double layer capacitors (EDLCs) and pseudocapacitors based on their mechanism of charge storage, EDLCs originates from the electrostatic charge diffusion and charge accumulation occurring at the electrode/electrolyte interface, whereas pseudocapacitors store charge through fast reversible faradaic reactions using metal hydroxides/oxides and conductive polymers as electrode materials, which can exhibit much higher specific capacitance and energy density than EDLCs.8–10Recently, there has been a lot of interest in flexible and lightweight supercapacitors for an increasing demand of emerging multifunctional portable, rolling-up and even wearable modern gadgets.11 These applications necessitate the supercapacitor electrodes with good electrochemical properties and high mechanical flexibility, thus typical flexible supercapacitors rely on well-integrated bendable freestanding film electrodes with soft materials as supporting substrates. Benefiting from the excellent conductivity and large surface area, carbon-based paper-like materials such as graphene paper, carbon nanotube paper and carbon cloth are primarily used as thin film electrode materials for flexible supercapacitors.12–14 However, the areal capacitance of these carbon-based paper materials is limited (<100 mF cm−2) because of the low packing density and large level of porosity.15,16 Furthermore, their poor mechanical properties, relatively high production cost and elaborate procedures would hinder their further practical application in supercapacitors. Therefore, these call for a quest to meet the need of flexible supercapacitors, which require electrodes with large areal mass, high areal performance and mechanically tough.
Under this circumstance, particular interest on constructing conductive polymer freestanding films has received notable attention since pseudo-capacitance electroactive materials show much higher special capacitance than carbon materials. Nevertheless, recent studies have mainly focused on improving the gravimetric performance, their areal or volumetric performance have to be further improved to fulfill their potential applications. Conductive polymer have been mostly assembled onto carbon-based film substrates by direct chemical polymerization or electro-polymerization approach for flexible electrodes, which usually result in poor areal capacitance due to the low mass loading.
To overcome this challenge, supporting substrates with porous structure such as celluloses represent a big opportunity. Celluloses possess the robust mechanical strength to withstand bending, folding and twisting, and can combine with conductive polymers to achieve electrical conductivity, promoting them as a good candidate for flexible supporting substrates.17–22 Bacterial cellulose, as an abundant and renewable natural cellulose, can be produced on nontoxic, environmentally friendly, low-cost, and industrial scale by fermentation of bacteria (Acetobacter xylinum, E. coli, etc.),23 consisting of three-dimensional (3D) porous network composed of ribbon-shaped ultrafine nanofibers with widths less than 100 nm.17,24 The excellent structure of the BC make it a valuable candidate for PANI polymerization for the fabrication of large mass loading flexible electrodes. However, the specific capacitance of PANI/BC conductive nanocomposite is unsatisfactory due to the relatively low cyclic stability and electrical conductivity. As such, a certain amount of graphene is introduced to achieve a 3D interconnected conductive networks and accommodate lightweight and flexible paper electrodes.
In this work, we report a facile and scale-up methods based on a combined methodology of in situ chemical polymerization and vacuum filtering, employing PANI/BC/GN conductive and freestanding film to resolve the low mass loading and packing density of flexible electrodes for supercapacitor. The obtained PANI/BC/GN combines the advantages of high pseudo-capacitive PANI, excellent conductive GN and BC flexible substrate with regular network of interconnected pore channels. It not only facilitates the electrons transport and ions dispersion throughout the inter-connective network readily, but also overcomes the aggregation of GN and PANI/BC within the 3D conductive paper. These advantages make the flexible PANI/BC/GN paper a suitable and promising electrode material, achieving high areal capacitance of 6.15 F cm−2 with large mass loading of 12.9 mg cm−2 at a current density of 1 mA cm−2. Symmetric supercapacitor coupled with this two hybrid papers exhibits high areal capacitance (1.93 F cm−2 at 0.25 mA cm−2) and energy density (0.17 mW h cm−2 at 0.1 mW cm−2).
2. Experimental
2.1 Material
BC suspension was prepared as the following steps: BC pellicles (Hainan Yide Industry Co. Ltd.) were washed thoroughly and cut into small pieces, then pulped with a mechanical homogenizer at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, afterward, the BC slurry was diluted into deionized water to achieve the BC suspension (3.5 mg mL−1). GN (1 mg mL−1) was synthesized using the modified Hummers' method according to the reported references.25,26
000 rpm, afterward, the BC slurry was diluted into deionized water to achieve the BC suspension (3.5 mg mL−1). GN (1 mg mL−1) was synthesized using the modified Hummers' method according to the reported references.25,26
2.2 Preparation of PANI/BC/GN composites paper
40 mL BC suspension and 0.5 mL aniline monomer (5.5 mmol) were fully dispersed in 39.5 mL HCl (2 M) aqueous solution, the mixture solution was first sonicated for 0.5 h and transferred into an ice water bath. Afterward, the oxidant ammonium persulfate (APS, 300 mg, 1.32 mmol) was dissolved into 30 mL 1 M HCl aqueous solution and then added into the above suspension dropwise with vigorous stirring for 2 h. The color changed from ivory to dark green with the introduction of the APS. After polymerization, 18 mL of GN dispersion was poured into the reaction solution, and then the obtained composites solution was drained on a nitro cellulose filter membrane (porous size of 0.22 μm) to form a uniform paper and washed with deionized water for several times. The film was dried in an oven at 55 °C for 8 h and then peeled off to get the PANI/BC/GN freestanding paper. The films were named PANI-L/BC/GN, PANI-M/BC/GN and PANI-H/BC/GN due to the various concentration of PANI for 0.3, 0.4 and 0.5 mL, and the molar ratio of PANI to APS is about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The loading mass of active materials (PANI/GN) were 7.8, 10.2 and 12.9 mg cm−2, respectively.
1. The loading mass of active materials (PANI/GN) were 7.8, 10.2 and 12.9 mg cm−2, respectively.
2.3 Measurements and characterization
The obtained products were characterized by the following techniques. The compounds and composites were investigated by Fourier transform infrared spectroscopy analyzer (FT-IR, PerkinElmer Spectrum 100 Model) and X-ray diffraction (XRD, Rigaku 2500) equipped with Cu Kα radiation (λ = 1.5406 Å). The morphology and microstructure of the samples were characterized through scanning electron microscope (SEM, Hitachi S-4800) and transmission electron microscope (TEM, JEM-2100 F). Electrochemical studies were conducted with a CHI660E electrochemical workstation. The prepared flexible PANI/BC/GN paper was directly used as the working electrode without binders and other additives. The single electrode was tested in a three-electrode system in 1 M H2SO4 aqueous solution with active carbon (AC) and Ag/AgCl as the counter electrode and reference electrode, respectively. The counter electrode was fabricated as following: firstly, 200 mL BC suspension (0.7 mg mL−1) was filtered via vacuum filtration using a 0.22 μm porous nitro cellulose membrane to form a BC film. Then, 8 mg AC and 3 mg GN dispersion ink were poured onto the prepared BC paper to form a hybrid membrane. Finally, the precipitate was dried at 60 °C for 8 h and automatically peeled off to get the free standing paper. Symmetric supercapacitor was measured in two electrode configuration using both flexible hybrid PANI-H/BC/GN paper as electrodes, which separated with cellulose acetate diaphragm.3. Results and discussion
The synthesis routes of PANI/BC/GN freestanding paper electrodes via a two-step process was depicted in Scheme 1. Here, a key design for flexible electrode is to employ an ultrafine 3D fibrous BC as flexible supporting substrate and achieve high mass loading and high areal capacitance. Firstly, PANI was grown directly on porous BC via a simple in situ polymerization method in acid circumstance to obtain PANI/BC conductive nanofibers suspension. The aniline hydrochloride could soak into the inner network of BC. Meanwhile, the abundant functional hydroxyl groups (hydroxyl, ether, ester group etc.) of BC (Fig. 1a) could interact with amine groups of aniline to develop hydrogen bands, guaranteeing the uniform distribution and polymerization of aniline. Secondly, to avoid the aggregation of PANI/BC and construct a uniform 3D architecture with high specific surface area, some graphene was incorporated under vigorous stirring after the end of polymerization to form the PANI/BC/GN freestanding electrodes (Fig. 1b). Furthermore, in an attempt to realize an optimum freestanding paper, experiments with different mass loading and corresponding electrochemical tests were performed through controlling various aniline monomer concentration using the same technique. The PANI/BC/GN paper (0.32 mm, Fig. 1c) serves as a flexible electrode in the absence of any other binders, and retains the BC flexibility after the growth of PANI and the introduction of GN, and can be bended to a large degree (Fig. 1d).The morphology and microstructures of the samples were first examined by SEM and TEM. The continuous nanofibers, smooth surface and three-dimensional porous network of BC paper can be identified in Fig. 2a. Fig. 2b presents a typical TEM image of the BC nanofibers with the diameter in the range from 20 to 60 nm. After polymerization of the PANI onto BC, the roughness of the BC fibers increases and the entire nanofiber surface (even for the junction points) are enclosed with PANI, achieving a porous and uninterrupted “skeleton/skin” architecture (Fig. 2c). Fig. 2d presents SEM image of the PANI-H/BC/GN film, in which GN uniformly disperse and connect with PANI/BC nanofibers, ensuring more conductive pathways and higher electrical conductivity. As shown in Fig. 2e, the PANI coating is composed of randomly orientated small PANI nanosheets along the nanofibers. However, some surface of BC is not completely covered by the PANI, which is harmful for the conductivity. Such drawback can be overcome by the incorporation of GN, which can be clearly observed in Fig. 2f. The cross-sectional SEM image in Fig. 2g of PANI-H/BC/GN shows its layered and porous structure, which is mainly caused by the flow assembly effect during filtration.27 The magnified SEM image in Fig. 2h directly reveal the uniform distribution of GN sheets between the PANI/BC nanofibers, which constitutes continuous electron conductive framework. The as-prepared PANI/BC/GN hybrid electrodes combine the high conductivity of GN, large specific surface area and flexibility of BC and the pseudocapacitance of PANI, forming a high areal capacitance, freestanding and mechanical tough paper electrode.
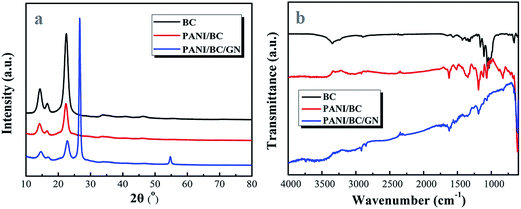
XRD patterns were further conducted to investigate the chemical bond and crystallization characteristics of the BC, PANI/BC and PANI/BC/GN, the results are shown in Fig. 3a. The three sharp peaks of BC centered at 14.3, 16.7 and 22.6° are related to the characteristic (110), (110), and (020) planes of cellulose I.28,29 While the PANI/BC shows a low intensity in comparison with BC, corresponding with the amorphous nature of the pure PANI.30 For PANI/BC/GN, the additional two peaks located at 22.6 and 54.7° confirm that the GN is incorporated successfully. Fig. 3b shows the FTIR spectra of BC, PANI/BC and PANI/BC/GN composites, and the results are in good agreement with previous studies. For pure BC, the characteristic broad peaks located at 3343, 2892 and 1052 cm−1 are ascribed to the O–H group, the asymmetrically stretching vibration of C–H and the anti-symmetric bridge stretching of C–O–C groups, respectively.31–33 While for PANI/BC, the strong peaks around 1628 and 1517 cm−1 are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of quinoid ring and benzenoid ring, respectively. Moreover, the characteristic peaks at 1341 and 1184 cm−1 originated from the stretching vibration of C–N secondary aromatic amine and C
C stretching vibration of quinoid ring and benzenoid ring, respectively. Moreover, the characteristic peaks at 1341 and 1184 cm−1 originated from the stretching vibration of C–N secondary aromatic amine and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. In addition, the new signal appeared at 829 cm−1 attributed to C–H out-of-plane bending vibration of the aniline ring. These FTIR results indicates the successful in situ polymerization of PANI/BC composites. After introducing the GN into the PANI/BC, the IR curve also hold the typical characteristic peaks of PANI/BC, which suggesting the GN is well-dispersed among the PANI/BC hybrid.
N. In addition, the new signal appeared at 829 cm−1 attributed to C–H out-of-plane bending vibration of the aniline ring. These FTIR results indicates the successful in situ polymerization of PANI/BC composites. After introducing the GN into the PANI/BC, the IR curve also hold the typical characteristic peaks of PANI/BC, which suggesting the GN is well-dispersed among the PANI/BC hybrid.
The electrochemical performance of the PANI/BC/GN electrodes were investigated in a three-electrode system in 1 M H2SO4. Fig. 4a compares the CV curves of the PANI/BC/GN paper electrodes conducted at 3 mV s−1. There are two pairs of well-defined redox peaks within −0.1 to 0.8 V on the CV curves of these electrodes, which are attributed to the doping/dedoping transitions of different PANI forms (the leucoemeraldine form and polaronic emeraldine form),17,34,35 indicating the strong pseudocapacitive behavior of the PANI. Obviously, the current response and integrated area in CV curves increase with the polymerization concentrations of PANI increase. The detailed CV behaviors of PANI-H/BC/GN flexible electrode were carried out at different scan rates from 1 to 40 mV s−1 (Fig. 4b). As the scan rate increases, a positive shift of oxidation peaks and a negative shift of reduction peaks can be observed, which is mainly due to the internal resistance. Fig. 4c shows the galvanostatic charge–discharge (GCD) curves of the PANI-H/BC/GN film at different current densities within a potential window from −0.1 to 0.7 V, implying good reversible pseudo-capacitance in a wide current range.
The specific capacitance can be calculated according to the formula, C = i × t/m(s) × V, where i is the discharge current, t is the discharge time, V is the potential, m is the loaded mass of the electroactive material and s is area. The areal capacitance and gravimetric capacitance of different PANI/BC/GN electrodes with an increase current densities are depicted in Fig. 4d and e. Obviously, the PANI-H/BC/GN flexible electrode has a higher areal capacitance than others at the same scan rate, indicating that the areal capacitance can be remarkably improved by the contribution of mass loading. An areal capacitance of 6.15 F cm−2 (477 F g−1 and 192 F cm−3) is achieved at 1 mA cm−2, which is higher than those reported conductive polymer membrane and at least one order of magnitude higher than that of carbon-based film. Table 1 lists the comparison of electrochemical performance of flexible electrodes.
| Flexible materials | Mass/mg cm−2 | Capacitance/mF cm−2 | Capacitance/F g−1 | Cycle performance | Ref. |
|---|---|---|---|---|---|
| 3D-RGO/PANI film | 525 | 385 (0.5 A g−1) | 88% after 5000 cycle | 37 | |
| CNT/PANI hydrogel | 680 (1 mA cm−2) | 38 | |||
| SWCNT/cellulose/PANI | 330 (0.2 mA cm−2) | 533 | 79% after 1000 cycle | 39 | |
| PANI/Ag/CNF | 0.82 | 176 (10 mV s−1) | 212 (10 mV s−1) | 40 | |
| PANI/PVA hydrogel | 4 | 2320 (1 A g−1) | 86% after 1000 cycle | 41 | |
| PANI/RGO film | 718 | 431 | 74% after 500 cycle | 42 | |
| PANI/cobalt-MOF/carbon cloth | 4 | 2146 (10 mV s−1) | 371 | 80% after 2000 cycle | 43 |
| PANI/RGO/graphite sheet | 1360 | 491 | 86% after 3000 cycle | 44 | |
| PANI/graphite nanosheets | 1 | 355.6 (0.5 mA cm−2) | 83 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycle 000 cycle |
45 | |
| PANI/BC/GN electrode | 12.9 | 6150 (1 mA cm−2) | 477 (1 mA cm−2) | 97% after 1000 cycle | This work |
| 56.3% after 8000 cycle |
The internal resistance, charge transfer kinetics, and ion diffusion process of PANI-L/BC, PANI-M/BC/GN and PANI-H/BC/GN film electrodes were investigated by electrochemical impedance spectroscopy (EIS). As shown in Fig. 4f, all the Nyquist plots are characterized by two parts: small semi-circle in the high frequency domain related to contact impedance generated by the PANI/BC/GN and electrolyte resistance, and in the low frequency domain, the vertical plots have larger slopes with respect to Z′ axis, which is the representative of ideal capacitance. The PANI-H/BC/GN electrode has the lowest first intercept with the X-axis and the smallest semicircle, suggesting a higher conductivity, lower charge transfer resistance and better ion response. In order to study the stability, the PANI-H/BC/GN electrode was tested by charge–discharge measurements for 8000 cycles at 50 mA cm−2, as shown in Fig. 4g, the specific capacitance increases with the increase of cycle number, nearly 107% of the maximum capacity is achieved after 300 cycles. After 8000 cycles, the capacity retains about 56.3%. PANI usually suffer poor cyclic performance caused by the swelling and shrinkage during the charge/discharge process. The “skeleton/skin” structure can inhibit the volumetric alternation of PANI to some extent and remains a well stability during cycling.
Symmetric supercapacitor is assembled by containing two PANI-H/BC/GN flexible electrodes separated by a cellulose acetate membrane in 1 M H2SO4 aqueous electrolyte. Fig. 5a presents the CV curves of the PANI-H/BC/GN symmetric supercapacitor at sweep rate between 1 and 20 mV s−1, indicating a capacitive behavior. Fig. 5b depicts the GCD curves of the device at different current densities from 1 to 10 mA cm−2 between 0 and 0.8 V. The quasi-triangular and symmetric shapes indicate the good reversibility and coulombic efficiency of the device.36 The capacitance is calculated from the GCD curves and the result is shown in Fig. 5c, the PANI-H/BC/GN symmetric supercapacitor has a high areal capacitance of 1.93 F cm−2 (29.7 F cm−3) at 0.25 mA cm−2 and 0.45 F cm−2 (6.92 F cm−3) at 10 mA cm−2. The electrochemical stability of the device was estimated through GCD test, and the device displays a cyclic stability with ∼53.6% of its initial capacitance retention over 5000 cycles at 8 mA cm−2 (Fig. 5d). Moreover, the areal power and energy density are two crucial characteristics to measure the practical applications, and depicted by Ragone plots in Fig. 5e. The supercapacitor delivers a maximum energy density of 0.17 mW h cm−2 at 0.09 mW cm−2, which is better than that of the most of recently reported conductive polymer flexible electrodes, and retains 0.03 mW h cm−2 at a maximum power density of 3.72 mW cm−2. Therefore, the flexible PANI-H/BC/GN film displays good electrochemical performance in both freestanding electrode and symmetric supercapacitor due to good integration of the porous BC substrate, high pseudo-capacitive PANI and excellent conductive GN, proving the potential application for flexible energy storage devices.
4. Conclusions
Freestanding and flexible PANI/BC/GN electrode with continuous 3D interconnected conductive networks is achieved in a facile “polymerization and filtering” strategy. The hybrid electrode paper has a high mass loading of 12.9 mg cm−2, and demonstrates desirable electrochemical performance with a high areal capacitance of 6.15 F cm−2 (477 F g−1). Furthermore, the symmetric supercapacitor offers a large areal capacitance (1.93 F cm−2) and energy density (0.17 mW h cm−2). This design provides a simple approach to fabricate high areal performance freestanding electrodes for flexible energy storage devices.Acknowledgements
The authors are grateful to the support of the Natural Science Foundation of China (Grant No. 21076050), Natural Science and Technology Support Program of China (Grant No. 2013BAE04B04), and Shenzhen BTR New Energy Materials INC.References
- Y. Huang, J. Liang and Y. Chen, Small, 2012, 8, 1805 CrossRef CAS PubMed
.
- Y. Sun, Q. Wu and G. Shi, Energy Environ. Sci., 2011, 4, 1113 CAS
.
- C. Zhang, W. Lv, Y. Tao and Q. H. Yang, Energy Environ. Sci., 2015, 8, 1390 CAS
.
- L. L. Liu, Z. Q. Niu, L. Zhang, W. Y. Zhou, X. D. Chen and S. S. Xie, Adv. Mater., 2014, 26, 4855 CrossRef CAS PubMed
.
- X. W. Yang, C. Cheng, Y. F. Wang, L. Qiu and D. Li, Science, 2013, 341, 534 CrossRef CAS PubMed
.
- H. H. Wang, E. W. Zhu, J. Z. Yang, P. P. Zhou, D. P. Sun and W. H. Tang, J. Phys. Chem. C, 2012, 116, 13013 CAS
.
- C. L. Long, D. P. Qi, T. Wei, J. Yan, L. L. Jiang and Z. J. Fan, Adv. Funct. Mater., 2014, 24, 3953 CrossRef CAS
.
- C. Z. Yuan, L. Yang, L. R. Hou, J. Y. Li, Y. X. Sun, X. G. Zhang, L. F. Shen, X. J. Lu, S. L. Xiong and X. W. Lou, Adv. Funct. Mater., 2012, 22, 2560 CrossRef CAS
.
- H. L. Cheng, A. D. Su, S. H. Li, S. T. Nguyen, L. Lu, C. Y. H. Lim and H. M. Duong, Chem. Phys. Lett., 2014, 601, 168 CrossRef CAS
.
- J. R. Miller and P. Simon, Science, 2008, 321, 651 CrossRef CAS PubMed
.
- Y. M. Sun, Z. Fang, C. X. Wang, K. R. R. M. Ariyawansha, A. J. Zhou and H. W. Duan, Nanoscale, 2015, 7, 7790 RSC
.
- J. Zhi, C. Y. Yang, T. Q. Lin, H. L. Cui, Z. Wang, H. Zhang and F. Q. Huang, Nanoscale, 2016, 8, 4054 RSC
.
- U. N. Maiti, J. Lim, K. E. Lee, W. J. Lee and S. O. Kim, Adv. Mater., 2014, 26, 615 CrossRef CAS PubMed
.
- Q. H. Meng, H. Wu, P. Y. N. Meng, K. Xie, Z. X. Wei and Z. X. Guo, Adv. Mater., 2014, 26, 4100 CrossRef CAS PubMed
.
- Y. T. Hu, C. Guan, G. X. Feng, Q. Q. Ke, X. L. Huang and J. Wang, Adv. Funct. Mater., 2015, 25, 7291 CrossRef CAS
.
- Y. Xu, Z. Lin, X. Huang, Y. Liu, Y. Huang and X. Duan, ACS Nano, 2013, 7, 4042 CrossRef CAS PubMed
.
- S. H. Li, D. K. Huang, B. Y. Zhang, X. B. Xu, M. K. Wang, G. Yang and Y. Shen, Adv. Energy Mater., 2014, 4, 1301655 CrossRef
.
- Z. J. Shi, G. O. Phillips and G. Yang, Nanoscale, 2013, 5, 3194 RSC
.
- G. Y. Zheng, L. B. Hu, H. Wu, X. Xie and Y. Cui, Energy Environ. Sci., 2011, 4, 3368 CAS
.
- H. Cheng, Z. Dong, C. Hu, Y. Zhao, Y. Hu, L. Qu, N. Chen and L. Dai, Nanoscale, 2013, 5, 3428 RSC
.
- Y. J. Kang, S. J. Chun, S. S. Lee, B. Y. Kim, J. H. Kim, H. Chung, S. Y. Lee and W. Kim, ACS Nano, 2012, 6, 6400 CrossRef CAS PubMed
.
- Y. P. Fu, X. Cai, H. W. Wu, Z. B. Lv, S. C. Hou, M. Peng, X. Yu and D. C. Zou, Adv. Mater., 2012, 24, 5713 CrossRef CAS PubMed
.
- F. L. Meng, L. Li, Z. Wu, H. X. Zhong, J. C. Li and J. M. Yan, Chin. J. Catal., 2014, 35, 877 CrossRef CAS
.
- Z. Schnepp, Angew. Chem., Int. Ed., 2013, 4, 1096 CrossRef PubMed
.
- X. Y. Dong, L. Wang, D. Wang, C. Li and J. Jin, Langmuir, 2012, 28, 293 CrossRef CAS PubMed
.
- L. N. Ma, H. J. Niu, J. W. Cai, P. Zhao, C. Wang, X. D. Bai, Y. F. Lian and W. Wang, Carbon, 2014, 67, 488 CrossRef CAS
.
- Q. Wu, Y. X. Xu, Z. Y. Yao, A. R. Liu and G. Q. Shi, ACS Nano, 2010, 4, 1963 CrossRef CAS PubMed
.
- K. Kafle, H. Shin, C. M. Lee, S. Park and S. H. Kim, Sci. Rep., 2015, 5, 15102 CrossRef CAS PubMed
.
- Z. Y. Wu, C. Li, H. W. Liang, J. F. Chen and S. H. Yu, Angew. Chem., Int. Ed., 2013, 52, 2925 CrossRef CAS PubMed
.
- A. Ansari, N. Parveen, T. H. Han, M. O. Ansarib and M. H. Cho, Phys. Chem. Chem. Phys., 2016, 18, 9053 RSC
.
- D. Adhikari, R. Oraon, S. K. Tiwari, J. H. Lee and G. C. Nayak, RSC Adv., 2015, 5, 27347 RSC
.
- L. Yuan, B. Yao, B. Hu, K. Huo, W. Chen and J. Zhou, Energy Environ. Sci., 2013, 6, 470 CAS
.
- S. H. Li, D. K. Huang, J. C. Yang, B. Y. Zhang, X. F. Zhang, G. Yang, M. K. Wang and Y. Shen, Nano Energy, 2014, 9, 309 CrossRef CAS
.
- J. J. Xu, K. Wang, S. Z. Zu, B. H. Han and Z. X. Wei, ACS Nano, 2010, 4, 5019 CrossRef CAS PubMed
.
- J. Yan, L. P. Yang, M. Q. Cui, X. Wang, K. J. Chee, V. C. Nguyen, V. Kumar, A. Sumboja, M. Wang and P. S. Lee, Adv. Energy Mater., 2014, 4, 1400781 CrossRef
.
- F. J. Miao, C. L. Shao, X. H. Li and K. X. Wang, J. Mater. Chem. A, 2016, 4, 4180 CAS
.
- Y. N. Meng, K. Wang, Y. J. Zhang and Z. X. Wei, Adv. Mater., 2013, 25, 6985 CrossRef CAS PubMed
.
- S. Zeng, H. Y. Chen, F. Cai, Y. R. Kang, M. H. Chen and Q. W. Li, J. Mater. Chem. A, 2015, 3, 23864 CAS
.
- D. T. Ge, L. L. Yang, L. Fan, C. F. Zhang, X. Xiao, Y. Gogotsi and S. Yang, Nano Energy, 2015, 11, 568 CrossRef CAS
.
- X. D. Zhang, Z. Y. Lin, B. Chen, W. Zhang, S. Sharma, W. T. Gu and Y. L. Deng, J. Power Sources, 2014, 246, 283 CrossRef CAS
.
- W. W. Li, F. X. Gao, X. Q. Wang, N. Zhang and M. M. Ma, Angew. Chem., 2016, 128, 9342 CrossRef
.
- M. Y. Kim, C. Lee and J. Jang, Adv. Funct. Mater., 2014, 24, 2489 CrossRef CAS
.
- L. Wang, X. Feng, L. T. Ren, Q. H. Piao, J. Q. Zhong, Y. B. Wang, H. W. Li, Y. F. Chen and B. Wang, J. Am. Chem. Soc., 2015, 137, 4920 CrossRef CAS PubMed
.
- G. X. Xin, Y. H. Wang, X. X. Liu, J. H. Zhang, Y. F. Wang, J. J. Huang and J. B. Zang, Electrochim. Acta, 2015, 167, 254 CrossRef CAS
.
- B. Yao, L. Y. Yuan, X. Xiao, J. Zhang, Y. Y. Qi, J. Zhou, J. Zhou, B. Hu and W. Chen, Nano Energy, 2013, 2, 1071 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |