Chiral separation by nonaqueous capillary electrophoresis using L-sorbose–boric acid complexes as chiral ion-pair selectors†
Lili Lvab,
Lijuan Wang*ab,
Yanan Zouab,
Rui Chenab and
Jiaojiao Yuab
aKey Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding 071002, China. E-mail: wanglijuan@hbu.edu.cn; Fax: +86 312 5971107; Tel: +86 312 5971107
bKey Laboratory of Medical Chemistry and Molecular Diagnosis, Ministry of Education, Hebei University, Baoding 071002, China
First published on 25th October 2016
Abstract
A chiral nonaqueous capillary electrophoresis (NACE) method using L-sorbose–boric acid complexes as the chiral ion-pair selectors was developed for enantioseparation of nineteen chiral analytes including eight β-blockers, seven β-agonists, two phenothiazines antihistamines, and two fluoroquinolone antibiotics. The effects of L-sorbose and boric acid concentrations, triethylamine concentration, applied voltage, and capillary temperature on the enantioseparation were systematically investigated. Under the optimized conditions, thirteen pairs of enantiomers were baseline resolved and five pairs of enantiomers were partially enantioseparated. Salbutamol showed a sign of separation with a shoulder peak. A reversal of propranolol enantiomer migration orders took place in the case when different concentrations of triethylamine were added into the NACE running buffers. The mass spectrometry (MS) results confirmed that triethylamine could promote the formation of negatively charged L-sorbose–boric acid complex chiral counter ions with different complex ratios. The enantioseparation results were compared with the previous work. Calibration curves showed excellent linearity with square of correlation coefficients (r2) greater than 0.9994 over concentration ranges of 5.0–500.0 μg mL−1 for each enantiomer of clenbuterol and esmolol, and 12.5–500.0 μg mL−1 for that of metoprolol. The limits of detection (LODs) and limits of quantitation (LOQs) for each enantiomer were 0.4–1.25 μg mL−1 and 1.5–4.0 μg mL−1, respectively. The relative standard deviations (RSDs) of intra-day and inter-day precisions of migration times were ≤1.64% (n = 6), and ≤6.54% (n = 15), respectively. The peak areas were ≤4.03% (n = 6), and ≤3.92% (n = 15), respectively. This method can likely be applied to the determination of each enantiomer in pharmaceutical formulations.
1. Introduction
It is widely acknowledged that the two enantiomers of chiral drugs usually display markedly different pharmacokinetic effects, biological interactions, pharmacological, and/or toxicological effects. For many racemic drugs, in most cases, the pharmacological activity is largely due to one of the enantiomers whereas the other enantiomer has either no effect or may lead to significant undesirable side effects.1,2 The administration of highly pure chiral drugs is important in the pharmaceutical industry and the marketing of single enantiomers of chiral drugs has become more common.3,4 Therefore, the development of analytical methods for chiral separations becomes very important for drug quality control, pharmacodynamic and pharmacokinetic studies, as well as toxicological investigations.1There is no doubt that enantioseparation by capillary electrophoresis (CE) has attracted great attention due to its high separation efficiency and flexibility.1,2,5–9 Among a variety of different CE modes, capillary zone electrophoresis (CZE), in which only a chiral selector is added into the general running buffer solution, are the most widely used for enantioseparations.5 While many CZE enantioseparations have used an aqueous solvent, nonaqueous capillary electrophoresis (NACE) has also been successfully applied for the analysis of chiral drugs.10–12 The viscosity, relative permittivity, apparent pH (pH*), electrostatic interactions, among other characteristics provide this method with entirely different chiral selectivity, solubility, separation efficiency and electric current ranges in relation to aqueous CZE.13 To date, some chiral selectors have been investigated in NACE, including cyclodextrins (CDs),7,14–18 macrocyclic antibiotics,12,19,20 chiral ligand-exchange complexes,21 chiral ion-pair complexes,22–27 and so on. However, compared with aqueous CE, the chiral selectors suitable for NACE is much fewer. It is significative to find novel chiral selectors in NACE to promote its development.
Ion-pair principle is one of the important chiral recognition mechanisms for the separation of charged chiral analytes in NACE.22,28 So far, some low molecular weight compounds, such as camphorsulfonates,22 L-gulonic acid,23 cinchona alkaloids and their derivatives24,25 have been used as ion-pair chiral selectors in NACE. Optical dialkyltartrates and polyols are chiral polyols, having cis-adjacent hydroxyl groups in their structures. They are very likely to react with boric acid to form new complexes of dialkyltartrate–boric acid or polyol–boric acid chiral complexes.27 In our previous work, various dialkyltartrate–boric acid complexes and four polyols–boric acid complexes showed good chiral recognition abilities for some chiral drugs in methanolic background electrolytes (BGEs). The chiral recognition mechanism was assumed to be ion-pair principle.27–30
D and L-sorboses were also easily obtained chiral polyols with cis-adjacent hydroxyl groups in their structures. D-Sorbose has been reported as the chiral selector for the separation of binaphthyl in aqueous CE, but no resolution obtained.31 And L-sorbose is much cheaper than D-sorbose, so the aim of this work is to evaluate the chiral recognition ability of L-sorbose in NACE for the enantioseparation of basic chiral drugs. In order to achieve good enantioseparation, the effects of L-sorbose and boric acid concentrations, triethylamine concentration, applied voltage, and capillary temperature on the enantioseparation were investigated. Mass spectrometry (MS) was also applied to confirm the formation of negatively charged L-sorbose–boric acid complex chiral counter ions. To validate the analytical performance and feasibility of this chiral NACE method for the determination of each enantiomer in pharmaceutical formulations, the linearity of calibration curve, intra-day and inter-day precisions, detection limit, and quantitation limit were studied.
2. Experimental
2.1 Instrumentation
NACE experiments were conducted on a TH-3100 high performance capillary electrophoresis system (Tianhui Institute of Separation Science, Baoding, China), equipped with a thermostatic system and a UV detector. Data were collected with a QIANPU (HW-2000) chromatography workstation. Uncoated fused silica capillary of 50 μm I.D. (Yongnian Reafine Chromatography Co., Ltd, Hebei, China) with a total length (Ltot) of 55.0 cm and an effective length (Leff) of 45.0 cm was used. The new capillary was conditioned by flushing with methanol for 10 min, 1.0 M NaOH solution for 20 min, distilled water for 5 min, 1.0 M HCl solution for 20 min and distilled water for 5 min in sequence. Before each run the capillary was rinsed with running buffer for 3 min. Samples were introduced into the capillary tube by positive pressure at 2.9 psi for 2 s. The experiments were performed at 25 ± 0.2 °C. The detection wavelength was set at 214 nm.MS experiments were performed with an Agilent 1100 series LC/MSD Trap XCT (Agilent Technologies, USA) equipped with an electrospray ionization source (ESI). The MS was controlled by Agilent software A.10.02. The experiments were performed in negative ESI-mode in a mass range of m/z 100–1000 with the following ionizer parameters: spray voltage 3000 V, nebulizer gas (N2) flow set at 15 psi, dry gas (N2) flow 5 L min−1 with a temperature of 325 °C. A spectral scan range of 100–1000 m/z with a maximum accumulation time of 200 ms and an ion charge control (ICC) target setting of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were applied. The sample was infused with a syringe pump at a flow rate of 3.0 μL min−1.
000 were applied. The sample was infused with a syringe pump at a flow rate of 3.0 μL min−1.
2.2 Chemicals and materials
Racemic sotalol hydrochloride, esmolol hydrochloride, clenbuterol hydrochloride, cycloclenbuterol hydrochloride, bambuterol hydrochloride, tulobuterol hydrochloride and clorprenaline hydrochloride were purchased from National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, Beijing, China). (S)-Propranolol hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA). The following racemic compounds were extracted by methanol from medicine tablets: propranolol hydrochloride (LI®, Tianjin Lisheng Pharmaceuticals Co., Ltd, China), metoprolol tartrate (BETALOC®, AstraZeneca Pharmaceuticals Co., Ltd, China), bisoprolol fumarate (BOSU®, Wellso Pharmaceuticals Co., Ltd, China), atenolol (YJ®, Beijing Yanjing Pharmaceuticals Co., Ltd, China), carvedilol (JUNENG®, Beijing Juneng Pharmaceuticals Co., Ltd, China), propafenone (LUMING®, Shandong Renhetang Pharmaceuticals Co., Ltd, China), salbutamol (LI®, Tianjin Lisheng Pharmaceuticals Co., Ltd, China), terbutaline sulphate (BRICANYL®, AstraZeneca Pharmaceuticals Co., Ltd, China), promethazine hydrochloride (YAODU®, Shanxi Linfen Jianmin Pharmaceuticals Co., Ltd, China), dioxopromethazine hydrochloride (QIANGSHEN®, Liaoning Dikang Pharmaceuticals Co., Ltd, China), lomefloxacin hydrochloride (BEIQING®, Hubei Kangzheng Pharmaceuticals Co., Ltd, China) and gatifloxacin (JIAKUISHA®, Baili Pharmaceuticals Co., Ltd, China).L-Sorbose (purity ≥ 98 wt%, water content ≤ 0.5 wt%) was purchased from Aladdin (Shanghai, China). Boric acid was the product of Baoding Chemical Reagent Factory (Baoding, China). Triethylamine (water content ≤ 0.2 wt%) was supplied by Tianjin Kermel Chemical Reagent Co., Ltd (Tianjin, China). Chromatographic-grade methanol (water content ≤ 0.02 wt%) was purchased from Tianjin Concord Technology Co., Ltd (Tianjin, China). The other reagents and chemicals were all of analytical reagent grade, made in China and used as received. Their water contents were all lower than 0.5 wt%.
2.3 Buffer and sample preparation
NACE running buffers were prepared by weighing the desired quantities of L-sorbose, boric acid, and dissolving them in methanol to the desired volume in a flask. The apparent pH (pH*) of the BGEs were adjusted by adding appropriate concentration of triethylamine to the running buffers.The sample solutions for NACE separation optimization were prepared by dissolving an appropriate quantity of each racemic sample in methanol to a concentration of 0.1 mg mL−1.
All of the solutions were filtered through a 0.22 μm syringe type filter prior to use.
3. Results and discussion
3.1 Effects of L-sorbose and boric acid concentrations on enantioseparation
It is generally acknowledged that the enantioseparation is based on temporary diastereomeric interactions between the chiral selector and enantiomers. Thus, the enantioselectivity can be greatly affected by the concentration of the chiral selector. Therefore, the effects of the concentration of L-sorbose and boric acid on the chiral separation were investigated in a range of 0–60 mM and 0–120 mM, respectively. The experimental results showed that no enantiomeric separation was observed for all the tested chiral analytes with the presence of L-sorbose or boric acid alone in these NACE systems. This indicated that L-sorbose and boric acid both played important roles for the enantioseparations and the real chiral selector resulting in the enantioseparation might be the complex of L-sorbose and boric acid. This is because L-sorbose molecule has cis-adjacent hydroxyl groups able to react with boric acid to form a complex acid, resulting in the enantioselectivity for the basic chiral analytes.As shown in Fig. 1, the resolutions for most of chiral analytes increased with the concentration of L-sorbose from 0 to 40 mM. When L-sorbose concentration increased from 40 to 60 mM, the resolutions for most of chiral analytes decreased. Finally, 40 mM L-sorbose was finally chosen to perform further investigations. As can be seen in Fig. 2, when the boric acid concentration was less than 40 mM, most of chiral analytes could not be resolved. The resolutions for most of the chiral analytes increased with the concentration of boric acid from 40 to 120 mM. When boric acid concentration was 100 mM, most of the chiral analytes could achieve good enantioseparations. When boric acid concentration was above 100 mM, the resolutions for most of the chiral analytes increased slowly. Finally, 100 mM boric acid was selected to perform further investigations.
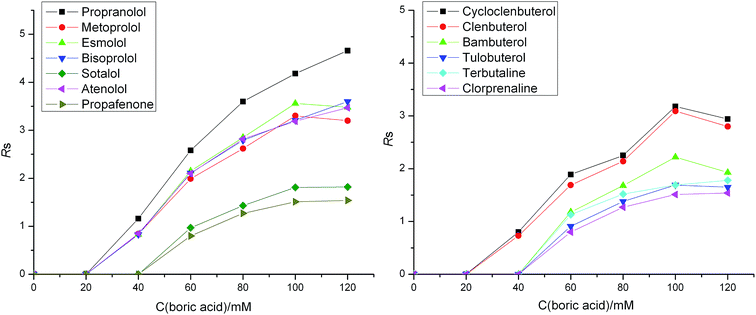 | ||
| Fig. 2 Effect of boric acid concentration on enantioseparation. Buffer component in addition to boric acid is 40 mM L-sorbose and 57.4 mM triethylamine in methanol. Other conditions are the same as in Fig. 1. | ||
The experimental results showed that the concentration of L-sorbose and boric acid all had great effects on the enantioseparation, and the reason could be explained from the following aspects. First of all, this may be because the reaction of L-sorbose and boric acid is reversible, the changes in the concentration of both of them will affect the production of the acid complex chiral selector and thus affect the chiral separation. Moreover, since L-sorbose and boric acid are both weakly acidic, the changes in the concentration of both of them will change the apparent pH (pH*) of BGE. Thus, the degree of ionization of the chiral analytes may be affected, thereby affecting the interactions between the chiral analytes and the chiral selector. The solubility of chiral selector, BGE of high viscosity, EOF of high intensity and other factors may also affect the enantioseparation.32
3.2 Effect of the concentration of triethylamine on enantioseparation
In NACE, the buffer solution acidity generally expressed by the apparent pH (pH*). In this study, the utilized chiral selectors were L-sorbose–boric acid complexes which were acidic protolytes, and the proper pH* was important for them to become negatively charged through deprotonation. Furthermore, the ions from the running buffer would give rise to a competing ion-pair formation with the selector and/or the analyte. Therefore, in order to avoid the interference of the other ions from the buffer, only triethylamine was added to the BGE to control the pH* value. The effect of triethylamine concentration on enantioseparation was investigated from 0 to 57.4 mM in BGE consisting of 60 mM L-sorbose and 100 mM boric acid, or 40 mM L-sorbose and 100 mM boric acid, respectively. Because the effects of triethylamine concentration on enantioseparation seemed relatively similar in these two buffer solutions, one of them was selected to be discussed. As shown in Fig. 3, all of the nineteen pairs of enantiomers had no separation in the BGE containing chiral selector without triethylamine. The resolutions for most of the chiral analytes increased firstly and then decreased with the increase of triethylamine concentration from 0 mM to 21.6 mM. When the triethylamine concentration increased from 21.6 mM to 57.4 mM, the resolutions for most of the chiral analytes increased. When 57.4 mM triethylamine was added to the BGE, most of the chiral analytes could be baseline separated. Therefore, 57.4 mM triethylamine was finally chosen to perform further investigations. | ||
| Fig. 3 Effect of triethylamine concentration on enantioseparation. Buffer component in addition to triethylamine is 40 mM L-sorbose and 100 mM boric acid in methanol. Other conditions are the same as in Fig. 1. | ||
It was assumed that triethylamine concentration affected the chiral separation mainly in three ways in this study. Firstly, with the change of triethylamine concentration, the pH* value of the BGE changed, affecting the dissociation of the acidic complex chiral selector. Secondly, the increase of triethylamine concentration also decreased EOF, increasing the difference in the migration times of the enantiomers,33 thus facilitated chiral separation. Finally, changes in triethylamine concentration might also affect the degree of the ionization of the chiral analytes, thereby affecting the interactions between the chiral analytes and the chiral selector.
3.3 Effects of applied voltage and capillary temperature on enantioseparation
In NACE, with the increase of applied voltage, the EOF increased and the analytical time decreased. However, the high voltage led to Joule heating and affected the separation of the analytes.34 Herein, the effect of applied voltage on the migration times, enantioselectivity (αeff), and resolution (Rs) was investigated at +10 kV, +15 kV, and +20 kV. However, the data of +20 kV was discarded due to the excessively messy peak shape. The results might be because the higher voltage would cause higher current and lead to more Joule heating. By increasing the applied voltage from +10 kV to +15 kV, the migration times of all of the chiral analytes decreased due to the increase of the EOF. Meanwhile, it was evident that the enantioselectivities and resolutions for most of chiral analytes increased when the applied voltage was +15 kV. To obtain a shorter analysis time and higher separation efficiency, +15 kV was finally selected.The capillary temperature is also a very important parameter. Changes in capillary temperature can cause variations in efficiency, viscosity, migration times, injection volumes, and detector response.35 In this study, the effect of capillary temperature was investigated at 20 °C, 25 °C, and 30 °C. The results showed that the migration times and enantioselectivity were not affected obviously by the capillary temperature. However, the resolutions increased with the increase of the capillary temperature from 20 °C to 25 °C. When the capillary temperature increased from 25 °C to 30 °C, the resolutions for most of the chiral analytes decreased. Thus, a capillary temperature of 25 °C was selected.
The optimized NACE conditions are concluded as follows: (1) buffer components: ① 100 mM boric acid, 60 mM L-sorbose, and 21.6 mM triethylamine in methanol (carvedilol); ② 100 mM boric acid, 60 mM L-sorbose, and 14.4 mM triethylamine in methanol (salbutamol, promethazine, dioxopromethazine, lomefloxacin, and gatifloxacin); ③ 100 mM boric acid, 40 mM L-sorbose, 57.4 mM triethylamine in methanol (the other thirteen analytes). (2) CE conditions: positive pressure injection for 2 s at 2.9 psi; applied voltage, +15 kV; detection wavelength, 214 nm; capillary temperature, 25 °C. The molecular structures of the nineteen chiral analytes and their enantioseparation under the optimized NACE conditions using L-sorbose–boric acid complex as the chiral selector are shown in Fig. 4.
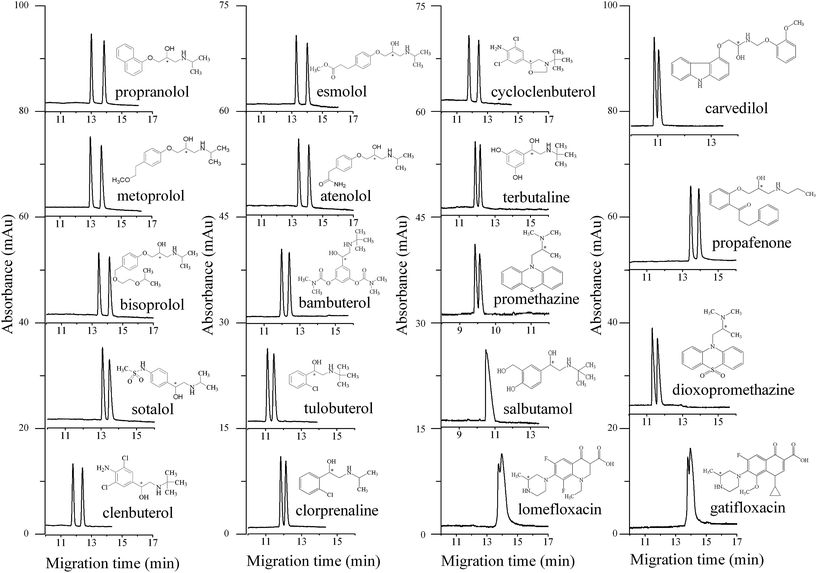 | ||
| Fig. 4 Enantioseparations of the nineteen chiral analytes under the optimized NACE conditions. Buffer component: 100 mM boric acid, 60 mM L-sorbose, and 21.6 mM triethylamine in methanol (carvedilol); 100 mM boric acid, 60 mM L-sorbose, and 14.4 mM triethylamine in methanol (salbutamol, promethazine, dioxopromethazine, lomefloxacin, and gatifloxacin); 100 mM boric acid, 40 mM L-sorbose, and 57.4 mM triethylamine in methanol (the other thirteen analytes). Other conditions are the same as in Fig. 1. | ||
3.4 Migration orders of propranolol enantiomers
Reversal of the migration orders of enantiomers is an important issue in chiral CE analysis. This is particularly true when one enantiomer is considered as an impurity which has to be detected and the mobility difference between the enantiomeric impurity and the major component is small.36 Furthermore, the marketing of single enantiomers of chiral drugs has become more and more common owing to the fact that the enantiomers of chiral drugs often vary in pharmacological and toxicological characteristics in many instances.28 Therefore, it is necessary to understand the migration orders of the enantiomers in order to prepare the desired single enantiomer. The identity of the peaks of propranolol enantiomers in all investigated buffer solutions was determined by spiking a single pure (S)-enantiomer into the solution of its racemic mixture. As shown in Fig. 5, the migration orders of propranolol enantiomers were reversed with the increase of triethylamine concentration in BGEs. The reason will be explained in Section 3.5.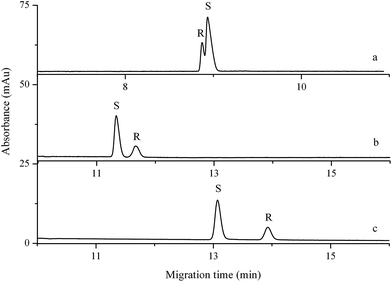 | ||
| Fig. 5 Migration orders of propranolol enantiomers with different concentrations of triethylamine. Buffer component: 100 mM boric acid, 60 mM L-sorbose, and 14.4 mM triethylamine (a), 39.6 mM triethylamine (b), or 57.4 mM triethylamine (c) in methanol. Other conditions are the same as in Fig. 1. | ||
3.5 Verification of the chiral separation mechanism
It was considered that the chiral separation mechanism in NACE was a reversible formation of diastereomeric ion-pairs between the negatively charged chiral counter ion L-sorbose–boric acid complex and the positively charged enantiomers. To verify this speculation, electrospray ionization mass spectrometry (ESI-MS, negative ionization mode) was used to characterize the structures of the complex chiral selector. NACE running buffers with different concentrations of triethylamine were directly used as the MS samples. As shown in Fig. S1(A),† MS sample without triethylamine obtained a spectrum with MS peaks at m/z 367.3, 407.1, and 447.0, which were consistent with the pseudo-molecular formula of [C12H20BO12 − H+]−, [C13H21B2O13 − H+]−, and [C14H22B3O14 − H+]−. Their complex ratios of L-sorbose and boric acid were 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively. No enantioseparation of basic analytes could be achieved with the same BGE in NACE. This was because there was not sufficient chiral selector to realize the chiral separation. As shown in Fig. S1(B) to S1(F),† the signal intensity of MS peaks at m/z 407.0 and 447.0 gradually increased with the increase of triethylamine concentration and the latter increased more. These results showed that triethylamine in the NACE running buffers could promote the formation of negatively charged L-sorbose–boric acid complex chiral counter ions with different complex ratios. In contrast to MS and NACE results, it was concluded that the negatively charged L-sorbose–boric acid complex counter ions with complex ratios of 2
3, respectively. No enantioseparation of basic analytes could be achieved with the same BGE in NACE. This was because there was not sufficient chiral selector to realize the chiral separation. As shown in Fig. S1(B) to S1(F),† the signal intensity of MS peaks at m/z 407.0 and 447.0 gradually increased with the increase of triethylamine concentration and the latter increased more. These results showed that triethylamine in the NACE running buffers could promote the formation of negatively charged L-sorbose–boric acid complex chiral counter ions with different complex ratios. In contrast to MS and NACE results, it was concluded that the negatively charged L-sorbose–boric acid complex counter ions with complex ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were the real chiral selectors in the NACE system. The former was the main chiral selector when lower triethylamine concentration existed in the buffers. Otherwise, the latter was the main chiral selector when higher triethylamine concentration existed in the buffers. And the latter chiral selector had better chiral recognition efficiency. This could also help to explain the reversal of migration orders of propranolol enantiomers.
3 were the real chiral selectors in the NACE system. The former was the main chiral selector when lower triethylamine concentration existed in the buffers. Otherwise, the latter was the main chiral selector when higher triethylamine concentration existed in the buffers. And the latter chiral selector had better chiral recognition efficiency. This could also help to explain the reversal of migration orders of propranolol enantiomers.
3.6 Comparison of enantioseparation using L-sorbose–boric acid complex and D-sorbitol–boric acid complex
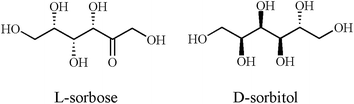
As reported in our previous work, the D-sorbitol–boric acid complex was used as chiral selector for the enantioseparations of six β-blockers and five β-agonists by NACE.29 Under the optimized conditions, baseline resolutions for clenbuterol and cycloclenbuterol were obtained; single peak appeared for tulobuterol and peak splitting for bambuterol, terbutaline, metoprolol, esmolol, bisoprolol, propranolol, sotalol and atenolol. In the current work, these six β-blockers and five β-agonists above were also chosen as the tested chiral analytes and they all achieved baseline enantioseparations under the optimized conditions.Compared with the L-sorbose–boric acid complex, the D-sorbitol–boric acid complex showed limited chiral recognition ability for the tested analytes. This might be related to the differences in the molecular structures between L-sorbose and D-sorbitol. As shown in Fig. 6, L-sorbose and D-sorbitol are all polyhydroxy compounds. The only difference in their molecular structures lies in the different groups on carbon two. Compared with L-sorbose, there is stronger polar group of hydroxyl group on carbon two in the molecular structure of D-sorbitol. This stronger hydrophilic group of hydroxyl group is near the cis-adjacent hydroxyl groups of D-sorbitol. It may have stronger interference with the reaction of D-sorbitol with boric acid to produce a certain number and purity of chiral selector.
3.7 Validation of the NACE method
In this study, upon optimization of the above-referenced separation conditions, validation of the NACE method was also performed according to ICH guidelines.37 The analytical performances were evaluated in terms of linearity, range, limit of detection (LOD), limit of quantification (LOQ), and precision. Clenbuterol, esmolol, and metoprolol were choosed as the tested analytes, recording electropherograms at 214 nm, 222 nm, and 224 nm, respectively.Calibration curves for each enantiomer were constructed at six concentration levels in the range of 5.0–500.0 μg mL−1 for each enantiomer of clenbuterol and esmolol, and 12.5–500.0 μg mL−1 for that of metoprolol. Good linearity was obtained for each enantiomer with square of correlation coefficient (r2) greater than 0.9994, and the slope and intercept were shown in Table 1. The calibration curves and linear equations were shown in Fig. S2.†
| Clenbuterol | Esmolol | Metoprolol | ||||
|---|---|---|---|---|---|---|
| Enantiomer 1 | Enantiomer 2 | Enantiomer 1 | Enantiomer 2 | Enantiomer 1 | Enantiomer 2 | |
| a NACE conditions are the same as in Fig. 4.b The concentration of each enantiomer was calculated as a half of its racemate. | ||||||
| Migration time intraday RSD (%) (n = 6) | 1.61 | 1.64 | 1.10 | 1.32 | 0.14 | 0.19 |
| Migration time interday RSD (%) (n = 15) | 4.08 | 4.39 | 6.34 | 6.54 | 3.96 | 3.95 |
| Peak area intraday RSD (%) (n = 6) | 3.15 | 2.83 | 2.11 | 2.37 | 2.91 | 4.03 |
| Peak area interday RSD (%) (n = 15) | 2.99 | 3.74 | 2.49 | 2.61 | 3.62 | 3.92 |
| Slope | 1424.4 | 1537.4 | 452.31 | 474.94 | 341.07 | 360.39 |
| Intercept | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 968 968 |
2413.5 | 2058.8 | 1544.7 | −86.003 |
| Correlation coefficient (r2) | 0.9996 | 0.9997 | 0.9995 | 0.9996 | 0.9996 | 0.9994 |
| LOD (μg mL−1) | 0.4 | 0.4 | 1.0 | 1.0 | 1.25 | 1.25 |
| LOQ (μg mL−1) | 1.5 | 1.5 | 3.0 | 3.0 | 4.0 | 4.0 |
Sensitivity of the method was evaluated by sequentially diluting sample solutions. LOD and LOQ of three racemic analytes were calculated at a signal-to-noise ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The LODs and LOQs for each enantiomer were 0.4–1.25 μg mL−1 and 1.5–4.0 μg mL−1, respectively (Table 1).
1, respectively. The LODs and LOQs for each enantiomer were 0.4–1.25 μg mL−1 and 1.5–4.0 μg mL−1, respectively (Table 1).
Intra-day precision was assessed by performing six parallel injections on the same day of solution containing 100 μg mL−1 racemic analyte, while monitoring peak areas and migration times for each enantiomer. The relative standard deviations (RSDs) of migration times were ≤1.64%, and that of peak areas were ≤4.03%, respectively. Inter-day precision was inspected by performing three parallel injections on five consecutive days at the same concentration level. The response functions were the same as described above, with migration time RSDs ≤ 6.54%, and peak area RSDs ≤ 3.92%, respectively (Table 1).
4. Conclusions
This paper firstly demonstrated the in situ synthesis of a novel type of complex chiral selector and its successful application in chiral NACE. The novel complex chiral selector had a good chiral recognition capability for most of the tested chiral drugs in NACE. Under the optimized conditions, thirteen pairs of enantiomers could be baseline resolved and partial enantioseparations were achieved for five pairs of enantiomers. Salbutamol showed a sign of separation with a shoulder peak. A reversal of enantiomer migration orders took place in the case when the apparent pH (pH*) was changed by adding different concentrations of triethylamine into the BGEs. The method was validated according to the proper ICH guideline and proved to be sensitive, and precise. This work is a significant advance in the application of polyhydroxy compounds having cis-adjacent hydroxyl groups as chiral selector in NACE.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC) (No. 21405031), the Natural Science Foundation of Hebei Province (No. B2015201160), the Science Foundation of Hebei University (No. 2013-258 and 2014-06).References
- G. Gübitz and M. G. Schmid, J. Chromatogr. A, 2008, 1204, 140–156 CrossRef PubMed.
- Z. Aturki, G. D'Orazio, A. Rocco and S. Fanali, Electrophoresis, 2011, 32, 2602–2628 CrossRef CAS PubMed.
- Z. S. Guo, H. Wang and Y. S. Zhang, J. Pharm. Biomed. Anal., 2006, 41, 310–314 CrossRef CAS PubMed.
- J. Znaleziona, I. Fejös, J. Ševčík, M. Douša, S. Béni and V. Maier, J. Pharm. Biomed. Anal., 2015, 105, 10–16 CrossRef CAS PubMed.
- W. A. Wan Ibrahim, S. R. Arsad, H. Maarof, M. M. Sanagi and H. Y. Aboul-Enein, Chirality, 2015, 27, 223–227 CrossRef CAS PubMed.
- S. Štĕpánová and V. Kašička, J. Sep. Sci., 2014, 37, 2039–2055 CrossRef PubMed.
- I. J. Stavrou, M. C. Mavroudi and C. P. Kapnissi-Christodoulou, Electrophoresis, 2015, 36, 101–123 CrossRef CAS PubMed.
- A. Amini, Electrophoresis, 2001, 22, 3107–3130 CrossRef CAS PubMed.
- Y. H. Ma, H. G. Zhang, H. L. Chen and X. G. Chen, Electrophoresis, 2014, 35, 3345–3354 CrossRef CAS PubMed.
- I. Ali, M. M. Sanagi and H. Y. Aboul-Enein, Electrophoresis, 2014, 35, 926–936 CrossRef CAS PubMed.
- S. Dixit and J. H. Park, Biomed. Chromatogr., 2014, 28, 10–26 CrossRef CAS PubMed.
- M. V. Lebedeva, A. F. Prokhorova, E. N. Shapovalova and O. A. Shpigun, Electrophoresis, 2014, 35, 2759–2764 CrossRef CAS PubMed.
- M. A. L. de Oliveira, B. L. S. Porto, C. de A. Bastos, C. M. Sabarense, F. A. S. Vaz, L. N. O. Neves, L. M. Duarte, N. da S. Campos, P. R. Chellini, P. H. F. da Silva, R. A. de Sousa, R. Marques, R. T. Sato, R. M. Grazul, T. P. Lisboa, T. d O. Mendes and V. C. Rios, Anal. Methods, 2016, 8, 3649–3680 RSC.
- A. C. Servais, M. Fillet, R. Mol, A. Rousseau, J. Crommen, G. W. Somsen and G. J. de Jong, Electrophoresis, 2010, 31, 1157–1161 CrossRef CAS PubMed.
- K. Lomsadze, A. Salgado, E. Calvo, J. A. López and B. Chankvetadze, Electrophoresis, 2011, 32, 1156–1163 CrossRef CAS PubMed.
- A. Rousseau, F. Gillotin, P. Chiap, E. Bodoki, J. Crommen, M. Fillet and A. C. Servais, J. Pharm. Biomed. Anal., 2011, 54, 154–159 CrossRef CAS PubMed.
- A. Rousseau, M. Pedrini, P. Chiap, R. Ivanyi, J. Crommen, M. Fillet and A. C. Servais, Electrophoresis, 2008, 29, 3641–3648 CrossRef CAS PubMed.
- A. C. Servais, A. Rousseau, G. Dive, M. Frederich, J. Crommen and M. Fillet, J. Chromatogr. A, 2012, 1232, 59–64 CrossRef CAS PubMed.
- G. F. Xu, Y. X. Du, B. Chen and J. Q. Chen, Chromatographia, 2010, 72, 289–295 CAS.
- A. P. Kumar and J. H. Park, J. Chromatogr. A, 2011, 1218, 1314–1317 CrossRef CAS PubMed.
- A. Karbaum and T. Jira, J. Chromatogr. A, 2000, 874, 285–292 CrossRef CAS PubMed.
- I. Bjørnsdottir, S. H. Hansen and S. Terabe, J. Chromatogr. A, 1996, 745, 37–44 CrossRef.
- Y. Carlsson, M. Hedeland, U. Bondesson and C. Pettersson, J. Chromatogr. A, 2001, 922, 303–311 CrossRef CAS PubMed.
- V. Piette, W. Lindner and J. Crommen, J. Chromatogr. A, 2002, 948, 295–302 CrossRef CAS PubMed.
- V. Piette, M. Lämmerhofer, W. Lindner and J. Crommen, J. Chromatogr. A, 2003, 987, 421–427 CrossRef CAS PubMed.
- M. Lämmerhofer, E. Zarbl, V. Piette, J. Crommen and W. Lindner, J. Sep. Sci., 2001, 24, 706–716 CrossRef.
- N. An, L. J. Wang, J. J. Zhao, L. L. Lv, N. Wang and H. Z. Guo, Anal. Methods, 2016, 8, 1127–1134 RSC.
- L. J. Wang, X. F. Liu, Q. N. Lu, G. L. Yang and X. G. Chen, J. Chromatogr. A, 2013, 1284, 188–193 CrossRef CAS PubMed.
- L. J. Wang, J. Yang, G. L. Yang and X. G. Chen, J. Chromatogr. A, 2012, 1248, 182–187 CrossRef CAS PubMed.
- L. J. Wang, S. Q. Hu, Q. L. Guo, G. L. Yang and X. G. Chen, J. Chromatogr. A, 2011, 1218, 1300–1309 CrossRef CAS PubMed.
- H. Nakamura, A. Sano and H. Sumii, Anal. Sci., 1998, 14, 375–378 CrossRef CAS.
- G. Blaschke and B. Chankvetadze, J. Chromatogr. A, 2000, 875, 3–25 CrossRef CAS PubMed.
- Y. Hedeland, J. Lehtinen and C. Pettersson, J. Chromatogr. A, 2007, 1141, 287–294 CrossRef CAS PubMed.
- N. Wang, Y. Y. Wu, X. Q. Wu, S. X. Liang and H. W. Sun, Anal. Methods, 2014, 6, 5133–5139 RSC.
- L. N. Xu, F. Luan, F. L. Hu, H. T. Liu and Y. Gao, Anal. Methods, 2013, 5, 762–766 RSC.
- H. Krajian, N. Mofaddel, D. Villemin and P. L. Desbène, Anal. Bioanal. Chem., 2009, 394, 2193–2201 CrossRef CAS PubMed.
- ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology, Q2 (R1), International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2005 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21806g |
| This journal is © The Royal Society of Chemistry 2016 |