Alkaline niobates ANbO3 (A = Li, Na, K) as heterogeneous catalysts for dipropyl sulfide oxidation†
Candelaria Leal Marchena*a,
Clara Sauxa,
Robinson Dinamarcab,
Gina Pecchib and
Liliana Pierellaa
aCentro de Investigación y Tecnología Química (CITeQ), UTN – CONICET, Maestro Marcelo López esq. Cruz Roja, (5016) Córdoba, Argentina. E-mail: cleal@frc.utn.edu.ar; Fax: +54 351 4690585; Tel: +54 351 4690585
bDepartment of Physical Chemistry, University of Concepción, Concepción, Chile
First published on 20th October 2016
Abstract
Alkaline ANbO3 (A = Li, Na, K) niobates with perovskite-type structure were prepared by a sol gel method, characterized by XRD, BET, TG/DTA, O2-TPD, DRS UV-Vis and FTIR and evaluated as heterogeneous catalysts for the selective oxidation of dipropyl sulfide (DPS) to its corresponding sulfoxide. The effects of different reaction parameters were evaluated, such as catalyst weight, the solvent and oxidant nature, reaction temperature and reuse. In order to gain better knowledge of the oxidation process, a kinetic study of the reaction was carried out. The global pseudo first order constant, the initial reaction rate and the apparent activation energy were calculated, and simplified reaction steps are proposed. KNbO3 showed the highest catalytic activity and sulfoxide selectivity with a periodic trend of KNbO3 > NaNbO3 ∼ LiNbO3.
1. Introduction
Perovskites are interesting inorganic structures, whose general chemical formula is typically ABX3, where A and B are cations of dissimilar sizes (the larger A-site usually is a rare earth metal and the B-site a first row transition metal) and X is an anion binding to both. Although this general formula is relatively simple, it hides an exceptional diversity of atomic arrangements. When X anion is oxygen, these materials are called oxide perovskites and have been actively studied for their ferro-electric, magnetic, piezoelectric, magnetoresistive and superconductive properties.1 For alkaline metals in the A-site, the charge balance to meet the perovskite structure is satisfied with vanadium(V) and niobium(V) at the B-site.2 Lithium niobate (LiNbO3), sodium niobate (NaNbO3) and potassium niobate (KNbO3), well-known as alkaline niobates, have been reported with interesting applications such as piezoelectricity, pyroelectricity, electro-optic, and nonlinear optical behavior.3 Moreover, interesting catalytic properties have also been reported,4,5 closely related to the atomic number of the metal.6,7 Lithium niobate is a well-known and transparent semiconductor with important technological applications mainly in solid-state based optical devices, such as optical modulators, in either bulk or nanostructured forms.6,8 Potassium niobate has also been widely reported as an interesting material for optical and photocatalytic applications.9 Moreover, KNbO3 and NaNbO3 have been identified as promising alternatives to currently used piezoelectric ceramics, mainly based on lead zirconate titanate. However, their use or the use of other niobates as catalysts in the selective oxidation of sulfides has been scarcely reported in literature.10,11 It have been reported in literature for sulfoxidation reactions employing mild oxidizing agents, the use of homogeneous catalyst, such as HAuCl4·4H2O,12 Mo(VI) salts,13 and heterogeneous catalysts, such as, MoO3;14 dioxo-molybdenum(VI) complex;15 heterogeneous TiO2;16 tantalum(V) and niobium(V);17 composite oxide catalyst LiNbMoO6,18 among others.The chemistry of organic sulfur-containing compounds has been widely reported due to presence of sulfoxides and other sulfur compounds as important intermediates in several pharmaceutical and fine chemistry reactions.19,20 The well-known omeprazole medicine and the fipronil pesticide are, among others, two typical examples of the extensive applications of these intermediates.21,22 Sulfoxides are generally prepared via oxidation of the corresponding sulfides using stoichiometric amounts of organic23,24 or inorganic reagents.25,26 Sulfide oxidation does not only have applications in sulfoxide or sulfone synthesis, since they can also be harnessed in decontamination processes, as sulfoxides are less toxic than the corresponding sulfides.27 Most of these experimental procedures are expensive, require drastic conditions, extended reaction times and low yields of the desired products are obtained. The use of “green oxidants” such as molecular oxygen or hydrogen peroxide in the sulfide to sulfoxide reaction is a very attractive, as these oxidants are readily available, inexpensive and environmentally benign with the formation of water as the only by-product.
This oxidation reaction employing hydrogen peroxide as oxidant has been shown to be one of the most attractive methods; however, the reaction rates are slow, therefore, to improve on this method, further studies have been undertaken.28–30
In light of the above, we hypothesize that with the advantages of the perovskite-type structure of alkaline niobates, it would be possible to obtain active heterogeneous catalysts for sulfide oxidation. To better understand the different activities of the alkaline niobates and to identify the key-parameters involved in the catalytic oxidation of sulfides employing alkaline niobates with the perovskite structure, we present in this article Li, Na and K niobates that have been prepared, characterized and evaluated for the selective oxidation of dipropyl sulfide. The global pseudo first order constant, the initial reaction rate and the apparent activation energy are also determined.
2. Experimental
2.1. Alkaline niobates synthesis
Alkaline niobates (LiNbO3, NaNbO3 and KNbO3) were prepared following the procedure reported by Wang et al.,31 employing the corresponding alkaline acetates, hydrogen peroxide (H2O2) and citric acid as starting materials. An aqueous solution of the alkaline acetate was mixed at room temperature with a stoichiometric amount of NbCl5 previously dissolved in H2O2 under constant stirring. Citric acid was added under constant stirring in a molar ratio of 3 of citric acid to metal cations. The solution was further heated to 80 °C until gels were formed. Finally, the gels were dried at 60 °C for 15 h and then were calcined at 600 °C for 10 h.2.2. Characterization
X-ray powder diffraction (XRD) patterns were obtained employing a Rigaku diffractometer with Ni-filtered CuKα radiation (λ = 1.5418 Å). Specific areas were determined using the BET method from the nitrogen adsorption isotherms at 77 K on a Micromeritics apparatus model ASAP 2010. The differential thermogravimetry (DTA/TG) experiments of the solids were carried out using a Netzsch 409 PC equipment. The thermogravimetric analyses were performed in a 11% O2/He gas mixture using a constant sample weight of 40 mg for all experiments. The studied temperature range was 20–1000 °C. Temperature-programmed desorption of oxygen (O2-TPD) experiments were performed in a TPR/TPD 2900 Micromeritics system with a thermal conductivity detector. The samples were exposed to oxygen for 1 h at 700 °C and then, were cooled at 20 °C. The atmosphere was changed to a helium flow and the sample was heated at a constant rate of 10 °C min−1, and the desorbed oxygen was monitored with a thermal conductivity detector. The oxygen desorbed TPD-MS experiments confirm that the evolved gas and the helium flow only contain oxygen. The diffuse reflectance spectra (DRS UV-Vis) of the materials were recorded using a UV-visible JASCO V 650 spectrophotometer, to which a diffuse reflectance chamber with a 50 mm diameter integrating sphere and internal Spectralon coating is attached, in the 200–800 nm wavelength range. Fourier-transform IR spectra (FTIR) were recorded in a Nicolet Magna-IR 550 instrument equipped with a quartz sample holder with KBr windows. The niobates were mixed with KBr to obtain a niobate–KBr ratio of 1/150.2.3. Catalytic activity
The catalytic selective oxidation of dipropyl sulfide (DPS) (97%, Sigma-Aldrich) was carried out in a glass flask reactor (25 cm3) with magnetic stirring immersed in a thermostated bath, equipped with a reflux condenser using a substrate/oxidant molar ratio (R) of 1. The catalytic tests were performed by evaluating different reaction conditions such as: reaction temperature, weight of catalyst, nature of the oxidizing agent (H2O2 and tert-butyl hydroperoxide) and nature of the solvent (acetonitrile (99.5%, Cicarelli), acetone (analytical grade, Aldrich), 2-propanol (99.5%, Aldrich), methanol (99.5%, Cicarelli), ethanol (99.5%, Cicarelli), 2-butanol (99.5%, Merck) and n-hexane (97%, Riedel de Haën)). Blanck experiments were carried out under the same reaction conditions but without catalyst in the reaction mixture. The reactions were monitored by taking aliquots of the reaction mixture at different reaction times. Prior to analysis, the catalyst was separated by filtration. Organic compounds were analyzed quantitatively by gas chromatography (Hewlett Packard HP-5890) with a methyl silicone capillary column (30 m × 0.32 mm i.d.) and a flame ionization detector (FID) and qualitatively by mass spectrometry GC-Mass (Shimadzu QP 5050 GC-17 A) using a HP-S (25 m × 0.2 mm i.d.) capillary column.3. Results and discussion
3.1. Characterization
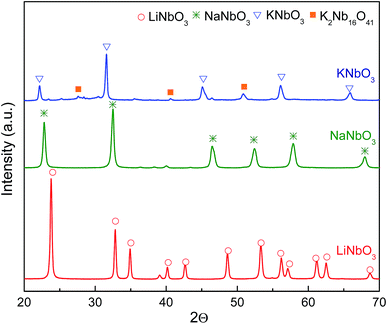
In order to identify crystalline phases in the synthesized materials, the X-ray diffraction patterns of the prepared materials are shown in Fig. 1. A rhombohedral structure with hexagonal axis (JCPDS 20-631) for LiNbO3 (ref. 32) can be observed, and for KNbO3, an orthorhombic perovskite-type structure (JCPDS 32-822) is noted with presence of K2Nb16O41 (JCPDS 14-287)33 as segregated phase. On the other hand, for NaNbO3 the diffraction profile corresponds to a single-phase orthorhombic perovskite-type structure (JCPDS 82-606).34For the main phase in each sample, the crystallite size was determined by the Scherrer's formula (d = Kλ/B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where d is the average size of the particles (considering spherical particles), K is a dimensionless shape factor with a value of 0.9, λ is the wavelength of X-ray radiation, B is the full width at half maximum of the diffracted peak and θ is the angle of diffraction. The obtained results, using the 2θ value of 42.5° (LiNbO3) and 58° (NaNbO3 and KNbO3) to calculate the crystallite size, are presented in Table 1. As it can be observed, lower and similar crystal sizes were obtained for NaNbO3 and KNbO3 and larger value for LiNbO3.
θ), where d is the average size of the particles (considering spherical particles), K is a dimensionless shape factor with a value of 0.9, λ is the wavelength of X-ray radiation, B is the full width at half maximum of the diffracted peak and θ is the angle of diffraction. The obtained results, using the 2θ value of 42.5° (LiNbO3) and 58° (NaNbO3 and KNbO3) to calculate the crystallite size, are presented in Table 1. As it can be observed, lower and similar crystal sizes were obtained for NaNbO3 and KNbO3 and larger value for LiNbO3.
| Catalysts | SBET (m2 g−1) | Crystalline phases | d (nm) | |
|---|---|---|---|---|
| LiNbO3 | 13 | Hexagonal rhombohedral | LiNbO3 | 40 |
| NaNbO3 | 17 | Orthorhombic | NaNbO3 | 18 |
| KNbO3 | 10 | Orthorhombic | KNbO3 K2Nb16O41 | 17 |
In Table 1 it is also reported the crystalline phases and the surface area obtained from nitrogen desorption isotherms. The lower SBET values show not a clear trend and are the expected values for these types of crystalline structures.35,36
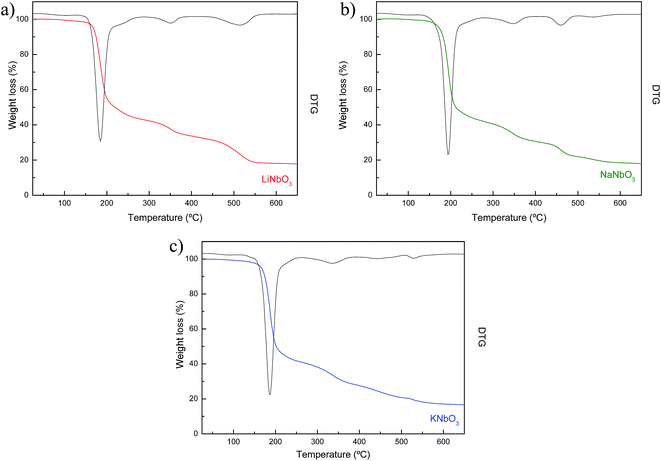
Fig. 2 shows the TG/DTG profiles of the prepared niobates from 25 °C to 650 °C. Although they were performed up to 1000 °C, no further changes were observed at higher temperatures, indicative of the crystallization of LiNbO3, NaNbO3 and KNbO3 structure26 also confirmed by XRD. Similar weight loss can be observed for all samples, and to identify the evolved products the evolution of 14, 18, 28, 32, 44 and 46 amu were followed by mass spectrometry (MS). The first weight loss about 3% between 30–100 °C corresponds to the removal of solvent in the samples and the second of approximately 60% below 200 °C to the loss of the free and bound water.37 At 340 °C corresponds the decomposition of the metal carboxylate gels and the final weight loss, between 400 °C and 500 °C, is assigned to the decomposition of residual organic groups.38
Considering that oxygen availability could improve DPS oxidation, O2-TPD patterns of the solids were also carried out (Fig. S1†). The alkaline niobates present only one broad oxygen desorption peak around 60 °C that could be ascribed to oxygen physically adsorbed on the surface.39 Physisorption implies a weak oxygen bond to the surface, and in order to optimize this property, the catalytic reaction temperature was fixed around this value. Due to TPD-MS experiments confirm that the evolved gas in the He flow only contains oxygen, it was possible to calculate the amount of oxygen desorbed for each niobate up to 200 °C from O2-TPD profiles (Table 2). Thus, an increase in the desorbed oxygen can be observed with the increase of the atomic number; and a large amount of desorbed oxygen could be associated with a larger catalytic activity.
| Desorbed oxygen (mmol m−2) | |
|---|---|
| LiNbO3 | 0.027 |
| NaNbO3 | 0.029 |
| KNbO3 | 0.033 |
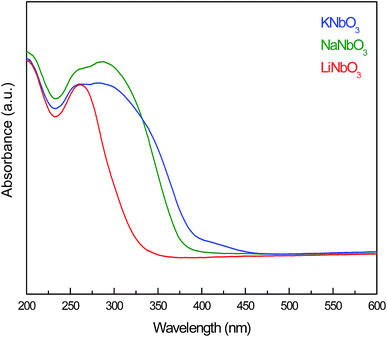
The coordination geometry and the chemical arrangement of the Nb species in the prepared niobates were evaluated by DRS UV-Vis spectroscopy. In Fig. 3 a clear maximum excitation at 260 nm, characteristic of the intrinsic niobate group, followed by a broad band at 300–400 nm can be observed. It is noticeable that the broad peak at higher wavenumber appears as a shoulder for KNbO3 and NaNbO3. This feature can be attributed to a slightly distorted NbO6 octahedra characteristic of perovskite-type oxides, as it has been previously reported by Turco et al.40 and Zhu et al.41 The difference in the DRS UV-Vis spectra of the KNbO3 and NaNbO3 perovskite-type oxides with regard to LiNbO3 can be explained by the differences in the Nb–O–Nb angle within their crystalline structures. A stronger delocalization of the electronic wave functions can be achieved in the 180° of the Nb–O–Nb angle in the orthorhombic perovskite structure of KNbO3 and NaNbO3 compared to the nearly 125° of the Nb–O–Nb angle in a rhombohedral LiNbO3 structure with a hexagonal axis.42
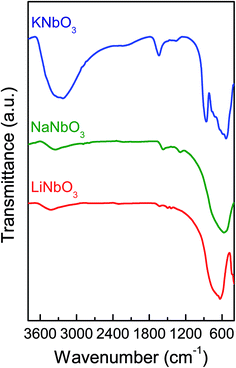
FTIR spectroscopy was employed to identify the nature of the segregated species present in the alkaline niobates and to verify the formation of the perovskite structure. The spectra of LiNbO3, NaNbO3 and KNbO3 (Fig. 4) show a band at ∼600 cm−1 attributed to the vibrational mode in the edge-shared NbO6 octahedra.43,44 In the case of KNbO3, a shoulder at 850 cm−1 was assigned to an asymmetric enlargement of the B–O bond of the octahedral BO6 structures indicative of the presence of the more crystalline perovskite structure. The band at 1495 cm−1 corresponds to the vibration of the CO32− groups45 and the band at 3600 cm−1 is assigned as the enlargement of the O–H bond in water. As it can be observed, LiNbO3 has the cleanest surface structure and KNbO3 the largest amount of segregated phases, identified as CO32− groups, also confirmed by XRD. In summary, the FTIR characterization provides strong evidence of crystalline phase formation for LiNbO3, NaNbO3 and KNbO3.
3.2. Catalytic activity
Table 3 shows the conversion (mol%) obtained in the catalytic oxidation of DPS by the Li, Na and K niobates after 60 min of reaction time. It was observed that KNbO3 has a higher degree of conversion followed by NaNbO3 and LiNbO3, indicating an increase in DPS oxidation down the alkaline group. The selectivity of DPS oxidation to prepare the sulfoxide (>95 mol%) is noticeably larger for these alkaline niobates, with sulfone (<5 mol%) as the only by-product observed. The larger catalytic activity of KNbO3 can be related to the formation of surface oxygen-containing complexes in nano-sized particles, as previously reported by Zhang et al.46 for K-substituted lanthanum perovskites. According to Escalona et al.47 the highly symmetric cubic perovskite structures, similar to the perovskite structure with A and B-site vacancies, does not improve the catalytic activity. The orthorhombic and rhombohedral structures are the most appropriate structures for perovskite-type oxides, i.e., as catalysts in catalytic combustion reactions.47 On the other hand, despite the different atomic number, Na and Li niobates showed similar catalytic activity. This behavior could be attributed to the amount of desorbed oxygen, which in both of these niobates is similar as shown in the O2-TPD results. Ivanov et al.48 have demonstrated that excess oxygen in the rhombohedral perovskite structure enhances the availability and reactivity of oxygenated species on the catalytic surface due to weaker oxygen binding in this structure. Thus, LiNbO3 may increase its catalytic activity due to the hexagonal rhombohedral structure, as demonstrated by XRD, equaling the activity of NaNbO3.| Catalysts | Conversion (mol%) | Selectivity (mol%) | Ea (kJ mol−1) | k (10−2) (min−1) | r0 (10−3) (mol g−1 min−1) | |
|---|---|---|---|---|---|---|
| Sulfoxide | Sulfone | |||||
| a Reaction conditions: sulfide (4.0 mmol), 35% (w/v) H2O2 (4.0 mmol). Solvent: acetonitrile. Reaction time: 60 min. Catalyst amount: 20 mg. Temperature: 60 °C. | ||||||
| LiNbO3 | 55.1 | 96.2 | 3.8 | 5.9 | 4.5 | 9.1 |
| NaNbO3 | 55.4 | 96.1 | 3.9 | 5.8 | 3.8 | 7.6 |
| KNbO3 | 77.1 | 94.9 | 5.1 | 3.0 | 5.5 | 11.0 |
To obtain a better correlation of the catalytic activity with the structural behavior of these materials, a kinetic study of the oxidation of DPS was performed. The suitable fit of the experimental data with a pseudo first order reaction, allowed us to determine the global pseudo first order constant and the initial reaction rate. The apparent activation energy (Ea) was calculated using the Stationary Point Method.49 The obtained values are listed in Table 3 and in all cases the correlation coefficients were higher than 0.9. The global constant (k) and initial reaction rate (r0) resulted similar for the LiNbO3 and NaNbO3 which agree with the catalytic behavior observed, and a higher value was obtained for the KNbO3, reflected in a higher final DPS conversion. The estimated apparent activation energy values are much lower than those reported by Trikalitis and Pomonis50 for perovskites in the reaction of propylene production. This important finding also supports the potential use of these alkaline niobates as catalysts for sulfide oxidation.
To determine the optimal reaction conditions, the most active niobate, KNbO3 was selected to evaluate different reaction parameters. Firstly, the effect of the catalyst amount was studied and the obtained results are presented in Fig. 5.
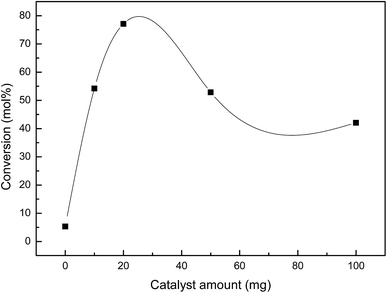 | ||
| Fig. 5 Effect of catalyst weight on DPS conversion for KNbO3 (reaction conditions: R = 1. Temperature: 60 °C. Reaction time: 60 min). | ||
At 60 min of reaction, DPS conversion values increases significantly from 54.0% to 77.1% when the catalyst mass increases from 10 to 20 mg. An increased concentration of KNbO3 decreases degree of conversion with almost no changes in the sulfoxide selectivity (>94%), indicative of a similar reaction mechanism.51 The optimal weight of catalyst can be explained considering the reports of Choudhary et al.52 and Qi et al.53 who indicate that at higher catalyst loading, hydrogen peroxide decomposition rates also increase, according to reaction (1).
| H2O2 → H2O + 0.5O2 | (1) |
Considering that H2O2 decomposition and DPS oxidation occur simultaneously, the decreased availability of the oxidant explains the decrease in conversion level when the catalyst loading is raised to 20 mg. The non-catalytic reaction carried out under similar reaction conditions indicates a very low conversion rate, obtaining a final conversion of 5 mol% after 60 min.
The nature of the solvent is important on the reaction outcome, reaction kinetics and product selectivity, as previously reported by Corma et al.54 The effect of the solvent was studied using KNbO3, the better catalyst for the oxidation of DPS, using different protic and aprotic solvents. The DPS conversion (mol%) as a function of the solvent dielectric constant shown in Fig. 6 indicates a close relationship of sulfide conversion with solvent polarity, with no dependence on the aproticity or proticity of the solvent. This behavior indicates an increase in substrate concentration at the catalytic surface as the polarity of the solvent increases, as previously reported by Saux and Pierella.55 The low conversion of DPS in n-hexane is due to the low miscibility of H2O2 in low polarity organic solvents.
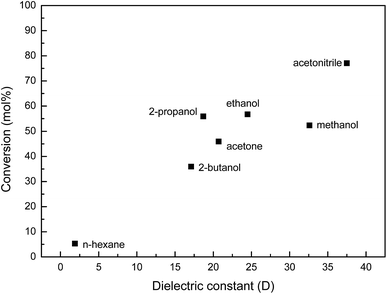 | ||
| Fig. 6 Effect of solvent nature on DPS conversion for KNbO3 (reaction conditions: R = 1. Catalyst amount: 20 mg. Temperature: 60 °C. Reaction time: 60 min). | ||
To study the effect of the nature of the oxidant, tert-butyl hydroperoxide (TBHP) was selected to compare with the environmental friendly and extensively used hydrogen peroxide. Under similar experimental conditions, it was found that using TBHP as the oxidant results in a DPS conversion level decrease from 77.1 to 11.9 mol% and the dipropyl sulfoxide (the product of interest) selectivity decreases from 94.9 to 83.8%. Moreover, considering that H2O2 produces only water and TBHP more toxic by-products, we confirmed that hydrogen peroxide is the most attractive and environmental friendly oxidant.
The effect of the reaction temperature on KNbO3 activity in DPS oxidation using a stoichiometric sulfide/oxidant molar ratio is shown in Fig. 7. A noticeable increase in conversion is observed up to 60 °C, and decreases at 80 °C. With regard to selectivity, at lower reaction temperatures the major product was sulfoxide; however, when the temperature was increased, the sulfoxide product decreased whereas sulfone production increased. At room temperature the DPS conversion was only 12.3% and increased to 43.4 and 77.1% as the temperature increased to 40 °C and 60 °C, respectively, with almost no change in sulfoxide selectivity (>94%). At 80 °C DPS conversion was slightly lower due to hydrogen peroxide consumption in a parallel auto-decomposition reaction (reaction (1)) that is favored at higher temperatures.
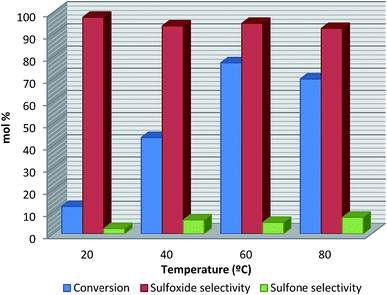 | ||
| Fig. 7 Effect of reaction temperature on DPS conversion for KNbO3 (reaction conditions: R = 1. Catalyst amount: 20 mg. Reaction time: 60 min). | ||
The recycled catalyst activity is an attractive property to reduce process costs and to follow environmental regulations. Again, KNbO3 was selected to evaluate the reusability of these materials. The recycling study was carried out by filtration of the catalyst after the reaction was finished; followed by calcination in air at 500 °C for 8 h to remove residual organic species that could be adsorbed on the catalyst surface. The catalyst recycling was carried out four times under the same reaction conditions, and the conversion (mol%) of DPS as a function of the cycle is shown in Fig. 8.
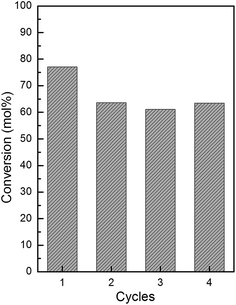 | ||
| Fig. 8 Reuse cycles for KNbO3 (reaction conditions: R = 1. Catalyst amount = 20 mg. Temperature: 60 °C. Reaction time: 60 min). | ||
A slight decrease is observed between the first and second reaction cycle with the conversion level remaining unchanged up to the fourth cycle. This behavior could be an evidence of leaching of some active species after the first use, a finding supported in a report by Mutreja et al.56 that shows that some active potassium species could leach from impregnated potassium catalysts. To evaluate this hypothesis, the oxidation with fresh catalyst was performed and after 10 min of reaction time, the catalyst was completely removed via filtration and the reaction system was left to continue reacting for 60 additional minutes. Afterwards, DPS conversion increases only in 13.4 mol%. This result confirms the previous hypothesis of active species lixiviation during the first use of the catalyst and there are responsible for the observed homogeneous behavior. Based on these results, it is proposed that some potassium species that have remained segregated post-KNbO3 synthesis are not embedded in the crystalline structure and could leach upon contact with a high dielectric constant solvent, such as acetonitrile, a solvent with a high dielectric constant that can solubilize ionic potassium species.
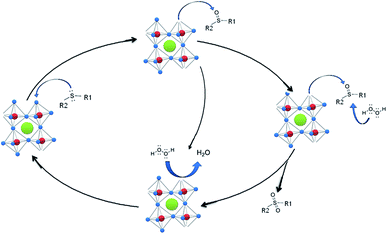
To gain a deeper insight of this catalytic oxidation and based on our interpretation of the experimental results and the identification of the oxidant products, simplified reaction steps are proposed for the catalytic DPS oxidation by KNbO3 (Scheme 1). It has been previously reported46,57 that the catalytic activity of perovskite-type catalysts for soot combustion is related to surface oxygen species, which migrate from within the perovskite structure to form surface oxygen-containing complexes in nano-sized particles. Based on this statement, the catalytic activity for DPS conversion can be related to these superficial oxygen species. We propose that the sulfur atom of the sulfide generates a nucleophilic attack to an electrophilic center producing the corresponding sulfoxide and an oxygen vacancy, as previously reported.11 Therefore, the large amount of desorbed oxygen obtained in the O2-TPD experiments can be associated with the highest catalytic activity for KNbO3, a perovskite-type oxide. The importance of desorbed oxygen from the surface of the catalysts for oxidation catalytic reactions was also analyzed by G. Pecchi et al.58 and Fino et al.59 Further, hydrogen peroxide restores the oxygen in the perovskite structure and generates water as by-product. The sulfoxide can be the final product, or generates the corresponding sulfone. The sulfone formation involves the interaction of the sulfoxide with the niobate by nucleophilic attack through the oxygen of the sulfoxide, by an H2O2 nucleophilic attack via a SN2 mechanism.60,61
4. Conclusions
All of the prepared alkaline niobates tested in this study show catalytic activity for sulfide oxidation in the presence of hydrogen peroxide as the oxidant. The observed activity depends mainly on the nature of the alkaline metal and is related to the formation of oxygen vacancies. The catalytic activity follows an atomic number dependent order, namely: KNbO3 > NaNbO3 ∼ LiNbO3. The global constant, reaction rate and apparent activation energy were determined and the obtained values agreed with the catalytic results. The highest catalytic activity was obtained for KNbO3, which is mainly attributed to the large amount of desorbed oxygen, and the amount of electron delocalization that increases as the Nb–O–Nb angle approaches 180° and promotes sulfide oxidation. The optimal experimental reaction conditions for DPS oxidation were at 60 °C with H2O2 as the oxidant, 20 mg of catalyst and acetonitrile as the solvent. Additionally, KNbO3 can be successfully used as an easily recyclable catalyst up to four times with minimal leaching of potassium species only during the first reaction cycle.Acknowledgements
This manuscript was partially supported by projects UTN-PID 1275 and UTI3864TC. Leal Marchena, Saux and Pierella thank CONICET-Argentina. Pecchi and Dinamarca thank CONICYT (Fondecyt Grant 1130005).References
- H. Mokbel, F. Dumur, B. Raveau, F. Morlet-Savary, C. Simonnet-Jegat, D. Gigmes, J. Toufaily, T. Hamieh, J. P. Fouassier and J. Lalevee, Tetrahedron, 2016 DOI:10.1016/j.tet.2016.03.057.
- G. Pecchi, C. Campos and O. Peña, Catal. Today, 2011, 172, 111–117 CrossRef CAS.
- C. Nico, T. Monteiro and M. P. F. Graça, Prog. Mater. Sci., 2016, 80, 1–37 CrossRef CAS.
- M. Manzo, F. Laurell, V. Pasiskevicius and K. Gallo, in Nano-Optics for Enhancing Light-Matter Interactions on a Molecular Scale, ed. B. D. Bartolo and J. Collins, Springer, Netherlands, 2013, pp. 421–422 Search PubMed.
- K. Wang and J. F. Li, J. Adv. Ceram., 2012, 1, 24–37 CrossRef CAS.
- B. R. Stanmore, J. F. Brilhac and P. Gilot, Carbon, 2001, 39, 2247–2268 CrossRef CAS.
- C. Badini, G. Saracco, N. Russo and V. Specchia, Catal. Lett., 2000, 69, 207–215 CrossRef CAS.
- R. Grange, F. Dutto and A. Radenovic, Niobates Nanowires: Synthesis, Characterization and Applications, Implement. Appl., InTech, 2011 Search PubMed.
- Q. P. Ding, Y. P. Yuan, X. Xiong, R. P. Li, H. B. Huang, Z. S. Li, T. Yu, Z. G. Zou and S. G. Yang, J. Phys. Chem. C, 2008, 112, 18846–18848 CAS.
- J. He, A. Xu, L. Hu, N. Wang, W. Cai, B. Wang, J. Hu and Z. Li, Powder Technol., 2015, 270, 154–162 CrossRef CAS.
- C. Saux, C. Leal Marchena, R. Dinamarca, G. Pecchi and L. Pierella, Catal. Commun., 2016, 76, 58–61 CrossRef CAS.
- Y. Yuan and Y. Bian, Tetrahedron Lett., 2007, 48, 8518–8520 CrossRef CAS.
- K. Jeyakumar and D. K. Chand, Tetrahedron Lett., 2006, 47, 4573–4576 CrossRef CAS.
- M. M. Khodaei, K. Bahrami and M. Khedri, Can. J. Chem., 2007, 85, 7–11 CrossRef CAS.
- I. Sheikhshoaie, A. Rezaeifard, N. Monadi and S. Kaafi, Polyhedron, 2009, 28, 733–738 CrossRef CAS.
- W. Al-Maksoud, S. Daniele and A. B. Sorokin, Green Chem., 2008, 10, 447–451 RSC.
- M. Kirihara, J. Yamamoto, T. Noguchi, A. Itou, S. Naito and Y. Hirai, Tetrahedron, 2009, 65, 10477–10484 CrossRef CAS.
- S. Choi, J.-D. Yang, M. Ji, H. Choi, M. Kee, K.-H. Ahn, S.-H. Byeon, W. Baik and S. Koo, J. Org. Chem., 2001, 66, 8192–8198 CrossRef CAS PubMed.
- F. Wang, C. Liu, G. Liu, W. Li and J. Liu, Catal. Commun., 2015, 72, 142–146 CrossRef CAS.
- H. M. Shen, W. J. Zhou, H. K. Wu, W. B. Yu, N. Ai, H. B. Ji, H. X. Shi and Y. B. She, Tetrahedron Lett., 2015, 56, 4494–4498 CrossRef CAS.
- M. Rahimizadeh, G. Rajabzadeh, S. M. Khatami, H. Eshghi and A. Shiri, J. Mol. Catal. A: Chem., 2010, 323, 59–64 CrossRef CAS.
- G. P. Romanelli, P. I. Villabrille, C. V. Cáseres, P. G. Vázquez and P. Tundo, Catal. Commun., 2011, 12, 726–730 CrossRef CAS.
- B. Zhang, S. Li, S. Yue, M. Cokoja, M. D. Zhou, S. L. Zang and F. E. Kühn, J. Organomet. Chem., 2013, 744, 108–112 CrossRef CAS.
- L. James, P. Martyn, S. Pandiaraju and A. K. Yudin, J. Organomet. Chem., 2000, 603, 98–104 CrossRef.
- D. H. R. Barton, W. Li and J. A. Smith, Tetrahedron Lett., 1998, 39, 7055–7058 CrossRef CAS.
- C. O. Kinen, L. I. Rossi and R. H. de Rossi, Appl. Catal., A, 2006, 312, 120–124 CrossRef CAS.
- N. M. Okun, J. C. Tarr, D. A. Hilleshiem, L. Zhang, K. I. Hardcastle and C. L. Hill, J. Mol. Catal. A: Chem., 2006, 246, 11–17 CrossRef CAS.
- G. P. Romanelli, G. P. Vázquez and P. Tundo, Synlett, 2005, 1, 75–78 Search PubMed.
- M. Kirihara, J. Yamamoto, T. Noguchi and Y. Hirai, Tetrahedron Lett., 2009, 50, 1180–1183 CrossRef CAS.
- A. Shaabani and A. H. Rezayan, Catal. Commun., 2007, 8, 1112–1116 CrossRef CAS.
- L. H. Wang, D. R. Yuan, X. L. Duan, X. Q. Wang and F. P. Yu, Cryst. Res. Technol., 2007, 42, 321–324 CrossRef CAS.
- S. Yao, F. Zheng, H. Liu, J. Wang, H. Zhang, T. Yan, J. Wu, Z. Xia and X. Qin, Cryst. Res. Technol., 2009, 44, 1235–1240 CrossRef CAS.
- N. Russo, S. Furfori, D. Fino, G. Saracco and V. Specchia, Appl. Catal., B, 2008, 83, 85–95 CrossRef CAS.
- N. M. Russo, D. Fino, G. Saracco and V. Specchia, J. Catal., 2005, 229, 459–469 CrossRef CAS.
- Y. Wang, J. Zhu, L. Zhang, X. Yang, L. Lu and X. Wang, Mater. Lett., 2006, 60, 1767–1770 CrossRef CAS.
- E. R. Camargo, M. Popa and K. Kakihana, Chem. Mater., 2002, 14, 2365–2368 CrossRef CAS.
- T. Ozawa, J. Therm. Anal., 1970, 2, 301–324 CrossRef CAS.
- T. Ozawa, J. Therm. Anal., 1975, 7, 601–617 CrossRef CAS.
- J. Zhu, Z. Zhao, D. Xiao, J. Li, X. Yang and Y. Wu, J. Mol. Catal. A: Chem., 2005, 238, 35–40 CrossRef CAS.
- R. Turco, A. Aronne, P. Carniti, A. Gervasini, L. Minieri, P. Pernice, R. Tesser, R. Vitiello and M. Di Serio, Catal. Today, 2015, 254, 99–103 CrossRef CAS.
- H. Zhu, Z. Zheng, X. Gao, Y. Huang, Z. Yan, J. Zou, H. Yin, Q. Zou, S. H. Kable, J. Zhao, Y. Xi, W. N. Martens and R. L. Frost, J. Am. Chem. Soc., 2006, 128, 2373–2384 CrossRef CAS PubMed.
- G. Blasse and L. G. J. De Haart, Mater. Chem. Phys., 1986, 14, 481–484 CrossRef CAS.
- N. Belmokhtar, R. Brahimi, R. Nedjar and M. Trari, Mater. Sci. Semicond. Process., 2015, 39, 433–440 CrossRef CAS.
- M. Tatsumisago, A. Hamada, T. Minami and M. Tanaka, J. Non-Cryst. Solids, 1983, 56, 423–428 CrossRef CAS.
- M. A. L. Nobre, E. Longo, E. R. Leite and V. Arela, J. Mater. Sci. Lett., 1996, 28, 215–220 CrossRef CAS.
- R. Zhang, N. Lou, B. Chen and S. Kaliaguine, Energy Fuels, 2010, 24, 3719–3726 CrossRef CAS.
- N. Escalona, S. Fuentealba and G. Pecchi, Appl. Catal., A, 2010, 381, 253–260 CrossRef CAS.
- D. V. Ivanov, L. G. Pinaeva, E. M. Sadovskaya and L. A. Isupova, Kinet. Catal., 2011, 52, 401–408 CrossRef CAS.
- B. Jankovic, B. Adnađević and S. Mentus, Chem. Eng. Sci., 2008, 63, 567–575 CrossRef CAS.
- P. N. Trikalitis and P. J. Pomonis, Appl. Catal., A, 1995, 131, 309–322 CrossRef CAS.
- M. R. Maurya, A. K. Chandrakar and S. S. Chand, J. Mol. Catal. A: Chem., 2007, 274, 192–201 CrossRef CAS.
- V. R. Choudhary, N. S. Patil, N. K. Chaudhari and S. K. Bhargava, Appl. Catal., A, 1995, 131, 309–322 CrossRef.
- B. Qi, X. H. Lu, D. Zhou, Q. H. Xia, Z. R. Tang, S. Y. Fang, T. Pang and Y. L. Dong, J. Mol. Catal. A: Chem., 2010, 322, 73–79 CrossRef CAS.
- A. Corma, P. Esteve and A. Martínez, J. Catal., 1996, 161, 11–19 CrossRef CAS.
- C. Saux and L. Pierella, Appl. Catal., A, 2011, 400, 117–121 CrossRef CAS.
- V. Mutreja, S. Singh and A. Ali, Renewable Energy, 2014, 62, 226–233 CrossRef CAS.
- Z. Li, M. Meng, F. Dai, T. Hu, Y. Xie and J. Zhang, Fuel, 2012, 93, 606–610 CrossRef CAS.
- G. Pecchi, B. Cabrera, E. J. Delgado, X. García and R. Jimenez, Appl. Catal., A, 2013, 453, 341–348 CrossRef CAS.
- D. Fino, N. Russo, G. Saracco and V. Specchia, J. Catal., 2003, 217, 367–375 CrossRef CAS.
- R. Frenzel, A. G. Sathicq, M. N. Blanco, G. P. Romanelli and L. R. Pizzio, J. Mol. Catal. A: Chem., 2015, 403, 27–36 CrossRef CAS.
- A. Chellamani and S. Harikengaram, J. Mol. Catal. A: Chem., 2006, 247, 260–267 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting figure of the oxygen desorption profiles of ANbO3 (A = Li, Na, K) niobates (Fig. S1). See DOI: 10.1039/c6ra21749d |
| This journal is © The Royal Society of Chemistry 2016 |