Modification of a porous oxide layer formed on an Al–Zn–Mg alloy via plasma electrolytic oxidation and post treatment using oxalate ions
Mosab Kaseem,
Jeong Hyeon Kwon and
Young Gun Ko*
School of Materials Science and Engineering, Yeungnam University, Gyeongsan 38541, Republic of Korea. E-mail: younggun@ynu.ac.kr; Fax: +82-53-810-4628; Tel: +82-53-810-2537
First published on 4th November 2016
Abstract
This study investigated the surface modification of the oxide layer of an Al–Zn–Mg alloy via plasma electrolytic oxidation (PEO) followed by post treatment using oxalate ions. The samples were treated by PEO in alkaline-aluminate electrolyte with and without Na2C2O4. The post treatment was performed additionally by immersing the PEO-treated sample into Na2C2O4 solution. Upon PEO, the existence of oxalate ions would minimize the structural micro-defects. This was attributed to the formation of an oxalate-adsorption thin-film, generating soft plasma sparks after breakdown. Moreover, the oxide layer after post treatment using Na2C2O4 solution exhibited the crack-free structure desirable for suppressing the corrosion rate.
1. Introduction
Plasma electrolytic oxidation (PEO) is an efficient and environmentally friendly surface treatment method based on plasma electrochemical reaction under a high voltage in a specific electrolyte to improve the corrosion resistance of valve metal alloys by fabricating oxide layers on their surfaces.1,2 One of the approaches to fabricate the oxide layer with a compact structure for achieving significant improvement of the corrosion-protection properties would be a surface modification by the alkaline electrolyte with the aid of the soluble organic compounds capable of controlling the plasma spark behavior during the PEO process.3–8 For example, Kaseem et al.4 reported that the addition of sodium benzoate to an alkaline-aluminate electrolyte led to the excellent corrosion resistance of 6061 Al because the compact oxide layer with a high fraction of α-Al2O3 was obtained via PEO. Indeed, Zhang et al.7 suggested that tannic acid could improve the corrosion resistance of the AZ91 Mg alloy because an insoluble Mg–tannate complex might be formed on the substrate surface, which led to an improvement in the micro-pore uniformity as well as increasing the thickness of the oxide layers. On the other hand, to promote the corrosion resistance of the oxide layers formed by PEO, it is essential to suppress the formation of the micro-defects in the oxide layer through post treatment in a suitable solution. For instance, Lu et al.9 reported that the corrosion resistance could be improved by using of poly-lactic acid to seal the micro-pores of the oxide layers formed on WE42 Mg alloy via PEO process.Oxalate ions have very low toxicity and behave as bidentate ligands for the formation of strong surface complexes with metallic ions, which could promote passivation of the metal surface, resulting in excellent corrosion-protection properties.10 Therefore, modification of the oxide layer using oxalate ions might be a novel way of developing the corrosion resistant Al alloys. Nevertheless, a lack of understanding of the fabrication of an oxide layer by adding oxalate ions as well as the corresponding corrosion properties was currently available. Therefore, the main aim of this study was two-fold: to investigate the influences of oxalate ions on the characteristics of the oxide layer during PEO, and to explore a post treatment using a Na2C2O4 solution to modify the outer layer of PEO coating on Al–Zn–Mg alloy in terms of its characteristics and corrosion behavior in 3.5 wt% NaCl solution.
2. Experimental procedures
The material used in this study was a Al–Zn–Mg alloy with a chemical composition of 5.1 Zn, 2.2 Mg, 1.2 Cu, 0.3 Fe, 0.2 Si, 0.2 Cr, 0.2 Mn and the balance Al in wt%. Prior to PEO, the sample with dimension of 25 mm × 20 mm × 2 mm were ground and polished with SiC abrasive paper to a girt of 1200 and then degreased in acetone. Aqueous solutions comprised of 4 g L−1 KOH, 3 g L−1 NaAlO2 without and with 1 g L−1 Na2C2O4 were called as Baths A and B, respectively, to produce plasma sparks and subsequent oxide layers. For both Baths, the values of pH and electric conductivity were approximately ∼13 and ∼19 mS cm−1, respectively. The PEO treatment was performed under alternating current conditions at a coating time, frequency, and current density were fixed to be 5 min, 60 Hz, and 50 mA cm−2, respectively. During the PEO coating process, the temperature of the electrolyte was maintained at 20 °C using stirring and cooling systems. To examine the influence of post treatment in solution containing oxalate ions, the oxide layer formed in Bath B was immersed in 10 mM Na2C2O4 (Bath C) for 60 min at ambient temperature. A scanning electron microscope (SEM-HITACHI, S-4800) equipped with energy dispersive spectrometer (EDS) was used to study the surface morphology and chemical composition of the elements in the oxide layers. The phase composition was examined by X-ray diffraction (XRD, RIGAKU, D-MAX 2500) with a step size of 0.05° and a scan range from 20 to 80°. To evaluate the corrosion behavior of the oxide layers, potentiodynamic polarization curves were assessed in aerated 3.5 wt% NaCl solutions using a coated sample with an exposed area of 1 cm2 as the working electrode, Ag/AgCl electrode as the reference electrode, and platinum plate as a counter electrode. After an initial delay of 30 min, the scans were conducted at a rate of 1 mV s−1 from −0.25 V to 0.4 V vs. the open circuit potential. For each sample, the electrochemical tests were carried out at least 3 times in order to insure the results reliability.3. Results and discussion
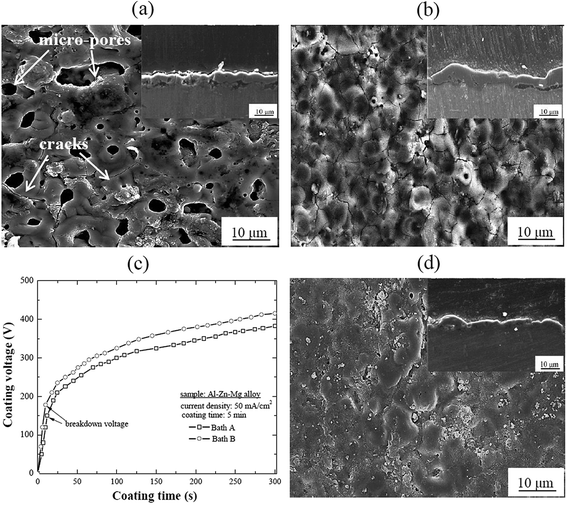
Fig. 1(a) and (b) show the representative surface morphologies of the oxide layers processed by PEO in the present electrolytes without and with Na2C2O4, denoting Baths A and B, respectively. Inset figures show the cross-sectional images of the oxide layers. The two oxide layers exhibited typical porous microstructure for the PEO process and some cracks were observed on the surface. The presence of the micro-pores within the oxide layer might be related to the appearance of sparks events during PEO whereas cracks were attributed to the rapid solidification of molten oxide in the relatively cool electrolyte.11,12 It was interesting that, when the sample was treated in Bath B, the micro-pores were less noticeable and some appreciable cracks were observed on the surface compared to those treated in Bath A. If an organic additive was added to an electrolyte during PEO, many oxygen bubbles would be adsorbed on the substrate surface and released easily from the oxide layer surface because this type of additive would lead to a change in the interfacial tension of the gas–liquid (oxygen bubble-electrolyte solution) and solid–liquid (oxide layer-electrolyte solution) by the formation of an adsorption film at the anode/electrolyte interface, resulting in the formation of an oxide layer with small micro-pores.5,13 However, the formation of a relatively compact microstructure in the sample processed in Bath B could enhance the final voltage during PEO (Fig. 1(c)), which would be an important factor that could affect the oxide layer properties, such as porosity and corrosion resistance. Interestingly, although the variation of electrolyte conductivity with addition of Na2C2O4 was minimal, the breakdown voltage of the sample coated in Bath B was higher by ∼25 V than that in Bath A. This difference would be attributed to the fact that the adsorption of C2O42− ions on the substrate surface would lead to an increase in the electrical resistance in the oxide layer, leading to a higher value of breakdown voltage in Bath B. Such results were consistent with the role of organic additives during PEO.7 According to Liu et al.,3 benzoate ions could adsorb easily on the surface of Mg alloy and form monolayer adsorption, which led to an increase in the electric resistance of Mg alloy as well as the generation of moderate sparks, facilitating the formation of a compact oxide layer. However, the formation of cracks in Bath B was attributed to the stress generated by the blocking/filling of the micro-pores which was associated with the addition of oxalate ions to the electrolyte and the formation of an oxalate-adsorption film on the surface of the oxide layer.14To ascertain how post treatment would affect the structural morphologies of the oxide layers, the sample treated by PEO in Bath B was immersed in 10 mM Na2C2O4 solution for 60 min at ambient temperature, which was designated as Bath B. Fig. 1(d) presents the surface morphology of the oxide layer processed in Bath B followed by post treatment in Bath C. It could be noted that the morphology of the oxide layer was influenced by the post treatment because no distinct cracks were observed and a relatively compact oxide layer was obtained.
Fig. 2 shows a series of schematic diagram to reveal the structural change in the oxide layers in order to clarify the influence of oxalate ions in Baths B and C in comparison to their counterpart in Bath A. A thin adsorption film formed directly on top of the substrate surface during PEO coating in Bath B containing C2O42− ions. C2O42− ions, as a kind of hard base, would be migrated toward the anode surface, so that the adsorption thin film formed prior to breakdown due to the chemical pairing between C2O42− and Al3+ (hard acid) from the substrate. Such adsorption film might retard the movement of water molecules, aluminate, and dissolved oxygen to the anode surface during PEO. Thus, soft sparks would be generated on the surface of the passivated oxide layer after breakdown, and the passivated oxide layer was reluctant to alter the fairly compact oxide layer due to the combined effects of breakdown, compound melting, and sintering. In addition, it is worth noting that C2O42− ions had also detrimental effects on the structure of the oxide layer since some cracks were observed in the sample processed in Bath B. Such cracks might be vulnerable sites for corrosive ions to penetrate and attack the substrate during a corrosion test. Hence, the post treatment in Bath C using C2O42− ions would be an effective sealing agent after PEO because post treatment in 10 mM Na2C2O4 solution led to the plugging of micro-pores and cracks in the oxide layer which resulted in the formation of a crack-free oxide layer as shown in inset cross-sectional images (Fig. 1). This was attributed to the ability of C2O42− ions to penetrate the micro-pores and cracks on the surface of the oxide layer by physical interlocking. Such behavior was observed in AZ91 Mg alloy treated with the PEO coating followed by post treatment using an organic sealing agent.15
EDS analysis was performed to examine the influence of oxalate ions on the chemical compositions of the oxide layers treated in Baths A, B, and C, as shown in Fig. 3(a). The oxide layers were composed mainly of Al and O elements. In addition, C element was detected in the oxide layer post treated in Bath C, suggesting that oxalate ions can be brought into most micro-pores and cracks in the oxide layer.
 | ||
| Fig. 3 EDS analysis and XRD patterns of the oxide layers treated in Baths A, B, and C (a) EDS analysis and (b) XRD pattern. | ||
To provide better insight into the compositions of the oxide layers, the XRD patterns are presented in Fig. 3(b). The oxide layer was composed mainly of γ-Al2O3 as well as Al corresponding to the substrate. The formation of γ-Al2O3 was likely favored due to the significant amount of Mg and Zn as the alloying elements in Al–Zn–Mg alloy with high cooling rate conditions.16,17 With the addition of Na2C2O4 to the electrolyte, the intensities of the γ-Al2O3 peaks became stronger while the intensity of the Al peaks decreased gradually, confirming that the oxide layer treated in Bath B was more compact than that in Bath A. However, despite of the formation of Al2(C2O4)3 was possible during the PEO, there was no evidence of any carbon-based crystalline phases in the XRD patterns. The lack of a sufficient concentration of oxalate ions in the present study would explain the absence of Al2(C2O4)3 on the oxide layer formed in Bath B. In addition, high temperature rise during PEO would lead to the decomposition of all organic compounds containing oxalate ions. On the other hand, for the oxide layer treated in Bath C, no carbon containing phase was identified in the XRD pattern, even though EDS analysis showed an appreciable amount of carbon in these oxide layers, indicating that oxalate ions tended to be incorporated into the oxide layer as an amorphous component rather than a crystallite compound.
To evaluate the effects of oxalate ions on the corrosion behavior of the oxide layers treated via PEO and the oxide layer after post treatment in an oxalate solution, potentiodynamic polarization curves were measured in 3.5 wt% NaCl solution and the results are displayed in Fig. 4. The electrochemical parameters, such as the corrosion potential (Ecorr), corrosion current density (icorr) and anodic/cathodic Tafel slopes (βa and βc) were derived from the test data. Based on the approximately linear polarization at the Ecorr, the polarization resistance (Rp) was determined,18 as listed in Table 1. In a typical polarization curve, a lower icorr corresponded to a lower corrosion rate and better corrosion resistance of the oxide layer. It could be seen from Table 1 that the corrosion resistance of the oxide layer was enhanced considerably when the micro-defects were sealed by the oxalate post treatment. In particular, the sample treated in Bath C showed a much lower icorr (6.95 × 10−10 A cm−2) than that with the oxide layer treated in Bath A (1.27 × 10−6 A cm−2) or the oxide layer treated in Bath B (4.80 × 10−8 A cm−2). The excellent corrosion-protection properties provided by post treatment in Bath C would be due to the beneficial effects of oxalate ions which played an effective role in blocking and eliminating micro-defects, such as micro-pores and cracks, leading to the suppression of the corrosion process by preventing the movement of corrosive ions towards the substrate during the corrosion test. Therefore, the post treatment of the PEO-treated sample would provide an efficient way to develop an oxide layer with superior corrosion performance.
| Sample | Ecorr (V) | icorr (A cm−2) | βa (V per decade) | βc (V per decade) | Rp (Ω cm2) |
|---|---|---|---|---|---|
| Bath A | −0.714 | 1.27 × 10−6 | 0.750 | 0.826 | 1.34 × 105 |
| Bath B | −0.643 | 4.80 × 10−8 | 0.179 | 0.215 | 8.83 × 105 |
| Bath C | −0.637 | 6.95 × 10−10 | 0.210 | 0.180 | 6.10 × 107 |
4. Conclusion
The influences of oxalate ions on the surface modification and corrosion behavior of the oxide layer formed on Al–Zn–Mg alloy by the PEO method followed by post treatment were examined systematically. Prior to post treatment, the microstructural results showed that oxalate ions would work as an organic additive during PEO, resulting in a relatively compact oxide layer with appreciable cracks. Interestingly, the oxalate ions would act as a sealing agent during post treatment where the conformal oxide layer was attained by eliminating the surface cracks that formed first during PEO. As a consequence, the oxide layer formed in the electrolyte containing 1 g L−1 Na2C2O4 followed by post treatment using an oxalate solution exhibited superior electrochemical performance to that without the oxalate sealing.References
- M. Kaseem, Y. H. Lee and Y. G. Ko, Mater. Lett., 2016, 182, 260–263 CrossRef CAS.
- T. S. N. Sankara Narayanan and M. H. Lee, RSC Adv., 2016, 6, 16100–16114 RSC.
- Y. Liu, Z. Wei, F. Yang and Z. Zhang, J. Alloys Compd., 2011, 509, 6440–6446 CrossRef CAS.
- M. Kaseem, M. P. Kamil, J. H. Kwon and Y. G. Ko, Surf. Coat. Technol., 2015, 283, 268–273 CrossRef CAS.
- D. Wu, X. Liu, K. Lu, Y. Zhang and H. Wang, Appl. Surf. Sci., 2009, 255, 7115–7120 CrossRef CAS.
- S. C. Yeh, D. S. Tsai, S. Y. Guan and C. C. Chou, Appl. Surf. Sci., 2015, 356, 135–141 CrossRef CAS.
- S. F. Zhang, R. F. Zhang, W. K. Li, M. S. Li and G. L. Yang, Surf. Coat. Technol., 2012, 207, 170–176 CrossRef CAS.
- G. Meng, F. Sun, Y. Shaoa, T. Zhang, F. Wang, C. Dong and X. Li, Electrochim. Acta, 2010, 55, 5990–5995 CrossRef CAS.
- P. Lu, L. Cao, Y. Lin, X. Xu and X. Wu, J. Biomed. Mater. Res., Part B, 2011, 96, 101–109 CrossRef PubMed.
- L. Kobotiatis, N. Pebere and P. G. Koutsoukos, Corros. Sci., 1999, 41, 941–957 CrossRef CAS.
- W. Ping, W. Ting, P. Hao and G. X. Yang, Mater. Lett., 2016, 170, 171–174 CrossRef CAS.
- Z. Qiu, Y. Zhang, Y. Li, J. Sun, R. Wang and X. Wu, RSC Adv., 2015, 5, 63738–63744 RSC.
- H. Guo and M. An, Thin Solid Films, 2006, 500, 186–189 CrossRef CAS.
- T. Narayanan, P. Song and M. Lee, Surface modification of magnesium and its alloys for biomedical application, Elsevier, New York, 1st edn, 2015 Search PubMed.
- H. Duan, K. Du, C. Yan and F. Wang, Electrochim. Acta, 2006, 51, 2898–2908 CrossRef CAS.
- V. Dehnavi, X. Y. Liu, B. L. Luan, D. W. Shoesmith and S. Rohani, Surf. Coat. Technol., 2014, 251, 106–114 CrossRef CAS.
- Y. J. Oh, J. I. Mun and J. H. Kim, Surf. Coat. Technol., 2009, 204, 141–148 CrossRef CAS.
- M. Stern and A. L. Geary, J. Electrochem. Soc., 1957, 104, 56–63 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |