Polymerized imidazolium ionic liquids crosslinking sulfonated poly(ether ether ketone) (SPEEK) for high-temperature proton exchange membrane
Abstract
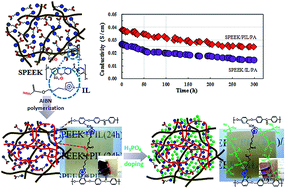
An ionic liquid (IL) monomer of (acryloyloxy)propanylimidazolium chloride with unsaturated carbon–carbon double bonds was synthesized. The IL monomer clung to sulfonated poly(ether ether ketone) (SPEEK) polymer owing to the electrostatic attraction between its cations and sulfonic groups. Polymerized ionic liquid (PIL) crosslinked with the SPEEK matrix after the in situ polymerization of the unsaturated carbon–carbon double bonds in the system of SPEEK/PIL. As a result, the SPEEK/PIL membrane displays a better performance in terms of tensile stress and chemical stability compared to those for the SPEEK/IL membrane. In order to improve the proton conductivity of the SPEEK/PIL membrane, phosphoric acid (PA) molecules were introduced through hydrogen bonds between imidazolium cations and phosphoric acid molecules. In the case of the SPEEK/PIL/PA membrane, a proton-conducting network was formed with more free and continuous phosphoric acid molecule chains owing to more phosphoric acid molecules being doped relative to the SPEEK/IL/PA membrane. The maximum proton conductivity of the SPEEK/PIL/PA membrane was 4.5 × 10−2 S cm−1 at 160 °C under anhydrous conditions and a value of 2.5 × 10−2 S cm−1 was even stable for more than 300 h at 140 °C. Moreover, the SPEEK/PIL/PA membrane possesses satisfactory mechanical properties, such as 2.64 MPa for tensile stress at 120 °C.

 Please wait while we load your content...
Please wait while we load your content...