Morphology evolution, formation mechanism and adsorption properties of hydrochars prepared by hydrothermal carbonization of corn stalk†
Abstract
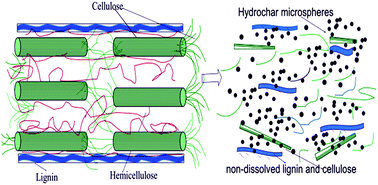
The formation condition, morphology evolution, reaction mechanism and adsorption properties of hydrochars prepared from corn stalk were explored using a hydrothermal method at low temperatures for a continuous period of reaction time. The experimental results showed that by prolonging the reaction time up to 26 h, corn stalk could be converted into hydrochar via HTC at a low temperature of 200 °C. It was found that hemicellulose was completely dissolved and more amorphous phases of cellulose and soluble lignin were fragmented and dissolved with increase of the reaction time. The ester, furfural and phenolic derivatives underwent a series of reactions in an aqueous solution to form polymerized hydrochar microspheres, whereas the non-dissolved cellulose and lignin went through a heterogeneous pyrolysis-like process to form polyaromatic char with an interconnected porous network structure. The as-prepared hydrochars with oxygen-containing functional groups and unique porous network structures could adsorb a larger amount of Cr(VI) than commercial activated carbon and were an effective and green sustainable adsorbent for the removal of Cr(VI) from an aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...