Open Access Article
Open Access ArticleMicrofluidic chip-based one-step fabrication of an artificial photosystem I for photocatalytic cofactor regeneration†
Xiaowen
Huang
ac,
Jian
Liu
 b,
Qingjing
Yang
d,
Yang
Liu
ac,
Yujiao
Zhu
ac,
Tenghao
Li
ac,
Yuen Hong
Tsang
ac and
Xuming
Zhang
*ac
b,
Qingjing
Yang
d,
Yang
Liu
ac,
Yujiao
Zhu
ac,
Tenghao
Li
ac,
Yuen Hong
Tsang
ac and
Xuming
Zhang
*ac
aDepartment of Applied Physics, The Hong Kong Polytechnic University, Hong Kong, P. R. China. E-mail: apzhang@polyu.edu.hk; Fax: +852-23337629; Tel: +852-4003258
bDepartment of Chemistry, Northwestern University, Evanston, Illinois 60208, USA
cThe Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen, P. R. China
dDepartment of Applied Biology and Chemical Technology, Hong Kong Polytechnic University, Hong Kong, PR China
First published on 11th October 2016
Abstract
We propose herein, a one-step method to assemble the immobilized artificial photosystem I (IAPSI) in a microfluidic chip, which integrated a preformed graphitic carbon nitride photocatalyst (g-C3N4) and electron mediator (M) in one chip and mimicked the characteristics of photosystem I. The simultaneous assembly of g-C3N4 and M could efficiently regenerate NADH from NAD+ under visible light irradiation, which verified the effectiveness of the assembly method. The in situ assembly method was thought to outperform traditional methods in several aspects in terms of facile synthesis, promotion of the combination of g-C3N4 and M through π–π stacking and an enhanced coenzyme regeneration rate. For comparison, we used the bulk g-C3N4–slurry and the few-layer g-C3N4–slurry system as the control to regenerate the photocatalytic cofactor/coenzyme NADH, and measured the required times of 305 s and 30 s, respectively, to accomplish 63% NAD+ conversion. In contrast, our IAPSI microreactor takes only 13 s, faster than the other two by factors of 23 and 2.3 times. Therefore, we assert that the simple, yet highly efficient nature of this technique can act as an important method for artificial photosynthesis, particularly in the photocatalytic cofactor recycling systems for the production of various valuable molecules.
1 Introduction
Solar energy is the most abundant energy on earth.1,2 One of the challenges for building a sustainable society is to develop practical materials and devices that can perform solar energy conversion (i.e., solar fuel or solar electricity).3–12 Natural photosynthesis is the fundamental process used by plants, algae and certain bacteria to harness energy from sunlight and convert it into chemical energy.13 In the light reaction of natural photosynthesis, through a series of photo-induced electron transfer processes in photosystem I (PSI), an integral membrane protein complex in photosynthesis, the solar energy (photons) is firstly transferred into active electrons and is then used to reduce the coenzyme nicotinamide adenine dinucleotide phosphate (NADP+) to NADPH.14,15 In view of the importance of NAD(P)H/NAD(P)+ in many biochemical conversions, particularly in many enzymatic redox reactions, mimicking such a process could provide a new way to design high-efficiency materials and devices for artificial photosynthesis.16–18The efficiency of photosynthesis in plants is based on the high surface-area-to-volume ratio structures of PSI (Fig. 1A) including a series of light-harvesting complexes, several metal–protein clusters and a group of redox biocatalysts, which are logically integrated in the phospholipid bilayer and confined to certain areas. These immobilized structural features not only facilitate efficient electron transfer and enhanced photon absorption, but also the isolation of the reaction substrates and products providing an ordered reaction chain. Thus, by mimicking the function of the PSI system, the regeneration of coenzymes (NADH, NADPH) through solar energy is a promising, sustainable and economic strategy that has attracted many research efforts that have contributed to important improvements.19–21 However, in the past, most of the work has focused on the heterogeneous photocatalysis for NADH regeneration, which renders the practical application impossible due to separation and recycling problems. Therefore, it is promising to develop a high-efficiency PSI artificial photosynthesis system on the immobilized system (IAPSI) for coenzyme regeneration, which would pave the way for practical uses in the near future.
Microfluidics, as versatile and promising systems, have several merits to benefit artificial photosynthesis in many ways.22 They have the advantages of large surface areas, short diffusion lengths and uniform irradiation. In addition, they have been shown to enable quick tests of photocatalysts, optimization of the operating conditions and rapid screening of various photocatalysts due to the merits of their wide tunability and parallel analysis.23,24 Moreover, compared with the conventional bulk slurry reactors that involve large reaction plates, microfluidics accommodates small volumes of fluids, resulting in low consumption of photocatalyst materials and samples.25 This is particularly important for rare or expensive reaction chemicals.
Metal-free efficient photocatalysts have attracted much attention in artificial photosynthesis,20,26,27 particularly the easily fabricated g-C3N4,7,28,29 which is an earth-abundant and low-cost photocatalyst obtained from heating melamine or urea. Importantly, the π–π stacking interaction between the adenine subunit of β-NAD+ and the electron-rich g-C3N4 promotes β-NAD+ to attach to the layered g-C3N4.15 Similarly, the π–π stacking interaction facilitates g-C3N4 to bind with the electron mediator, pentamethylcyclopentadienyl rhodium bipyridine {M, [Cp*Rh(bpy)Cl]Cl}, improving the transfer of electrons from the photocatalyst to the electron mediator. All of these advantages prompted us to choose g-C3N4 as the photocatalyst to fabricate the immobilized artificial PSI. Conventional methods to fabricate the IAPSI usually involve at least three steps: (1) the immobilization of the photocatalyst,17,19,30 (2) the synthesis of an electron mediator M, and (3) the introduction of a suitable mediator to the reaction system. In fact, the presence of an excessive mediator usually harms the enzyme activity thereby decreasing the reaction efficiency.31 Oppelt et al. proposed a smart and simple way to synthesize an IAPSI system immobilized on glass beads by coupling a light-harvesting ligand [2,2′-bipyridyl-containing poly(arylene-ethynylene)-alt-poly(arylene-vinylene)], with an organometallic rhodium complex for photocatalytic NAD+/NADH reduction.32 The NADH regeneration rate was only 21% at 26 h. Therefore, a system with a higher regeneration rate was required.
Herein, based on the merits of microfluidics, we proposed a simple one-step method in the microfluidic chip to simplify the processes and to improve the regeneration rate.25,33 In this method, the typical three steps for the formation of the artificial PSI were integrated into one, thereby saving processing time. In addition, this method also possesses other advantages such as (1) facile synthesis, which reduces the dependence on many types of instruments, (2) promotion of the π–π stacking interaction because M is formed and stored with the existing g-C3N4, increasing the chance of a π–π stacking interaction, and (3) a higher coenzyme regeneration rate than that of the slurry method. All of these merits proved this method useful, efficient and with significant improvements.
2 Materials and methods
2.1 Synthesis of few-layer g-C3N4
For few-layer g-C3N4 synthesis, we firstly synthesized the bulk g-C3N4 powder according to a procedure described in a previous paper.7 In detail, dicyandiamide (Aldrich, 99%) was heated at 550 °C for 4 h in static air with a ramp rate of 2.3 °C min−1. The cooling rate was kept at around 1 °C min−1. The resultant yellow agglomerates were milled into powder in a mortar. Then, through thermal oxidation etching of the bulk g-C3N4, few-layer g-C3N4 was prepared.34 A 400 mg amount of the bulk g-C3N4 was placed in an open ceramic container followed by a heating process at 500 °C for 2 h with a ramp rate of 5 °C min−1. Few-layer g-C3N4 was obtained as a light yellow powder.2.2 Material characterization
The FTIR spectrum was collected using a Vertex 70 FTIR spectrometer (Bruker Corporation). XRD measurements were performed on a D8 Diffractometer from Bruker Instruments. The TEM images were obtained from a TEM grid to perform high-resolution morphology characterization using a JEM2100F TEM system operating at 200 kV. The atomic force microscope (AFM) image was obtained by the MFP-3D (Asylum Research). The photoluminescence (PL) spectra were measured by a F-4600 fluorescence spectrometer (Hitachi, Japan) with an excitation wavelength of 365 nm (room temperature). The UV-vis absorbance spectra were recorded using a UV2550 spectrophotometer (Shimadzu Scientific Instruments). The electron mediator structure was determined by nuclear magnetic resonance analysis (300 MHz, Bruker, Germany).2.3 Microfluidic device fabrication
The PDMS layer with a simple leaf-like pattern (i.e., the narrowest part is 400 μm and the widest part is 4200 μm with a height of 40 μm) was fabricated using the standard soft lithography technique.35,36 In detail, we firstly designed a leaf-like photomask and obtained the corresponding SU-8 mold with selective exposure of UV-light. Then, we poured the uncrosslinked PDMS (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the mold. After a degassing and heating process (80 °C, 10 min), the PDMS layer with a leaf-like pattern was prepared.
1) on the mold. After a degassing and heating process (80 °C, 10 min), the PDMS layer with a leaf-like pattern was prepared.
2.4 IAPSI fabrication
The IAPSI fabrication process simply consisted of two steps, as shown in Fig. 1B. Step one involved mixing pentamethylcyclopentadienylrhodium(III) chloride dimer, 2,2′-bipyridyl and g-C3N4 together in ethanol. Thus, M [Cp*Rh(bpy)Cl]Cl was formed with g-C3N4. In step two, ethanol was removed by evaporation at 50 °C. The remaining part was IAPSI containing photocatalysts and electron mediator. Based on the merits of the microfluidic chip, we performed the photocatalytic cofactor regeneration in a microfluidic channel. Fig. 1C shows the simple IAPSI-microreactor fabrication process including the formation of IAPSI and covering the PDMS slide with a leaf-like pattern on it. This final fabricated microfluidic chip (40 μm high) is shown in Fig. 1D where the inset illustrates the leaf-like shape of g-C3N4–M.The traditional way to synthesize M [Cp*Rh(bpy)Cl]Cl was to reflux a mixture of RhCl3·3H2O and one equivalent of 1,2,3,4,5-pentamethylcyclopentadiene in methanol for 24 hours. The resulting red precipitate was filtered and suspended in methanol. On addition of two equivalents of 2,2-bipyridine, the suspension cleared up and a yellowish solution was formed. The remaining oily red crystals were extracted with chloroform and the solution was dried over anhydrous magnesium sulphate. After evaporation under reduced pressure, the residue was recrystallized from chloroform–benzene. The product was dissolved in methanol and with the addition of two equivalents of 2,2′-bipyridine. Then, [Cp*Rh(bpy)Cl]Cl was precipitated by the addition of diethyl ether and the final compound was dissolved in water turning to [Cp*Rh(bpy)H2O]2+. Obtaining electrons from photocatalysts, [Cp*Rh(bpy)H2O]2+ allows the simultaneous transfer of 2e− and 1H+ to NAD+, resulting in the formation of NADH (i.e., photocatalytic cofactor regeneration).
2.5 NADH photoregeneration
Photoregeneration of NADH was carried out by filling the microfluidic channel with 4 μL of reaction medium irradiated with a xenon lamp (300 W) through a 420 nm cut-off filter, avoiding the UV light-induced damage of the enzyme. The reaction medium (pH 8.0) was composed of NAD+ (0.2 mM), TEOA (15 w/v%), and phosphate buffer (100 mM). A 4 μL aliquot of the reaction medium was filled into the prepared microfluidic chip. 20 μg of g-C3N4 was immobilized in each chip and the complex M was equal to 0.25 mM.3 Results and discussion
3.1 Few-layer g-C3N4 characterization
We characterized the few-layer g-C3N4 by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), UV-vis absorption spectrometry, transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Fig. 2A shows the FTIR spectrum of the few-layer g-C3N4. The broad peaks between 3000 and 3500 cm−1 are attributed to the N–H band. The peaks at 1251, 1325, 1419, 1571, and 1639 cm−1 correspond to the typical stretching modes of C–N heterocycles. The peak at 810 cm−1 corresponds to the characteristic breathing mode of triazine units, which is compliant with the reported data.37 The few-layer g-C3N4 gives two XRD peaks at 12.7° and 27.8°, separately (Fig. 2B). The former one is the low-angle reflection peak, stemming from the lattice planes parallel to the c-axis and the later one is due to the stacking of the conjugated aromatic system. The UV-vis absorption spectrum of the few-layer g-C3N4 is broad, ranging from UV light to visible light (see Fig. 2C). The blue line in Fig. 2C is at the position of 420 nm, on the right of which can be seen the visible light absorption ability of g-C3N4. From the Tauc plot, the optical band gap of this g-C3N4 can be determined to be 2.9 eV from the (αhν)2versus hν graph, in which α, h and ν are the absorption coefficient, the Planck constant, and the light frequency, respectively. The optical image of the IAPSI is shown in Fig. 2D, and the corresponding enlarged structure by SEM is shown in Fig. 2E, from which we found that the few-layer g-C3N4 has a loose morphology. The TEM image (Fig. 2F) shows the graphene-like appearance of the few-layer g-C3N4, which is similar to previously published work.34 As an important factor, the thickness of the few-layer g-C3N4 was measured using AFM and found to be about 1.6 nm, corresponding to about three layers of g-C3N4 (Fig. S1†).3.2 Characterization of the electron mediator M
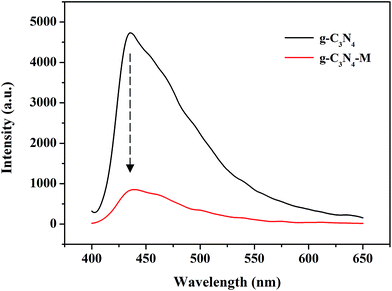
We compared the structural/chemical information of M in the traditional method and the one-step method by NMR. We obtained the correct structure in both methods, as shown in Fig. S2.† However, the one-step method saved time and reduced the chance to obtain impurities to some extent. The activity of the electron mediator M is of great importance. The reported method to detect the activity of M was the existence of the absorption peak at ∼230 nm, corresponding to the bipyridine ligand.38 Herein, we synthesized a series of M by the one-step method with different concentrations from 0.008 to 0.08 mM. As shown in Fig. 3A, the characteristic peaks at 232 nm, 304 nm and 312 nm are clear, proving the correct structure and the effective activity of the synthesized M. We then fitted these data and obtained good linearities between the M concentration and the absorption at the characteristic peaks (Fig. 3B). The correlation coefficient (R2) in each line was around 0.999, and the slope was 37.68, 17.74 and 17.65 mM−1 for the peaks at 232 nm, 304 nm and 312 nm, respectively. We also obtained very similar UV-vis spectra after storage in the fridge for three months, showing good stability for M stored in ethanol. The line of 340 nm in Fig. 3A is not for M, instead it indicates the position of absorption peak of NADH. From Fig. 3B, it is seen that the absorption at 340 nm varies very little with an increase in the concentration of M.After testing the activity of M, the coupling between g-C3N4 and M should also be verified because this is another critical factor that affects the catalytic performance. The interaction between g-C3N4 and M enables the hydride transfer from the excited conduction band of g-C3N4 to NAD+via the mediation of M. To directly detect and prove the electron transfer from g-C3N4 to M, we performed the PL assay. As shown in Fig. 4, a strong PL emission peak at 436 nm is observed for the few-layer g-C3N4 (black line), which can be attributed to the recombination of photo-induced electrons and holes. In contrast, the PL emission peak intensity of the few-layer g-C3N4 with M (red line) is much weaker than the former one, which directly proves an enhancement of the electron transfer from the g-C3N4 to M in the photocatalytic process.
3.3 NADH regeneration
We detected the NADH regeneration in three systems, which were the bulk g-C3N4–slurry system, the few-layer g-C3N4–slurry system and the IAPSI microreactor. The former two were used as the control to compare with the last one. The slurry systems were placed into a reflux condenser reactor equipped with a stirring bar and illuminated with a xenon lamp. The IAPSI microreactor was filled with the reaction solution and covered by a glass slide to avoid the water evaporation through the PDMS layer without stirring. Under similar conditions, previous studies in the microfluidic chip39 reported a decrease in the regeneration rate with an increase in the flow rate. To avoid the influence of flow rate, we choose the static flow (i.e., the flow rate is 0) in this work. Thus, the flow shear stress was not an influential factor. However, for the completeness of this research, we performed the shear stress test and presented the results in the ESI (see Fig. S3†). The distance between the IAPSI microreactor and the xenon lamp was fixed at 10 cm. Before the illumination, the reaction solution was placed in a dark environment for a sufficiently long time to achieve the adsorption–desorption equilibrium. Under the illumination, the concentration of NADH was calculated by measuring the absorption of the diluted reaction system at 340 nm. NADH had the peak absorption at 340 nm with an extinction coefficient of 6220 M−1 cm−1. Fig. 5A shows the UV-vis spectrum changes of the bulk g-C3N4–slurry system within 4 hours, whereas Fig. 5B shows the data of the few-layer g-C3N4–slurry system over 90 min. Although the times are different, the regeneration rate reaches similar levels of 50% at 90 min (few-layer g-C3N4–slurry) and 47.9% at 4 hours (bulk g-C3N4–slurry). Thus, the regeneration rate in the few-layer g-C3N4–slurry system is much faster, showing good catalytic ability. This can be attributed to its porous structural feature, which contains more sites that are reactive and improves the light harvesting by inner scattering, leading to more photogenerated electron carriers. These electrons are subsequently transferred to M, and then shuffled to NAD+ to regenerate 1,4-NADH.In the IAPSI microreactor, the regeneration rate was much more satisfactory. As shown in Fig. 5C, the absorption at 340 nm rises to 10.8% at 3 min and 55.9% at 30 min. To compare the regeneration rate in the three systems (bulk g-C3N4–slurry, few-layer g-C3N4–slurry and IAPSI microreactor), we plotted the regeneration percentage as a function of time in Fig. 5D. The exponential relationship is y = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp
exp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (−x/tc) + y0, where tc is the characteristic chemical time, defined as the time required for the concentration of A to fall from its initial value to a value equal to 1/e times the initial value. Calculated from Fig. 5D, the tc values of the IAPSI microreactor, the few-layer g-C3N4–slurry system and the bulk g-C3N4–slurry system are 13 s, 30 s and 305 s, respectively, which means the IAPSI microreactor is about 2.3 times and 23 times faster than the others. The improvement of the regeneration rate is ascribed to three reasons: (1) high surface-area-to-volume ratio, (2) short diffusion length and (3) uniform irradiation in the IAPSI microreactor. In the future, we will study more factors that affect the regeneration results, such as oxygen, flow rate and temperature by integrating more control units into the IAPSI microreactor. Furthermore, we will try to scale up the microreactor and work with other enzymes for the production of high performance functional materials.40–43
(−x/tc) + y0, where tc is the characteristic chemical time, defined as the time required for the concentration of A to fall from its initial value to a value equal to 1/e times the initial value. Calculated from Fig. 5D, the tc values of the IAPSI microreactor, the few-layer g-C3N4–slurry system and the bulk g-C3N4–slurry system are 13 s, 30 s and 305 s, respectively, which means the IAPSI microreactor is about 2.3 times and 23 times faster than the others. The improvement of the regeneration rate is ascribed to three reasons: (1) high surface-area-to-volume ratio, (2) short diffusion length and (3) uniform irradiation in the IAPSI microreactor. In the future, we will study more factors that affect the regeneration results, such as oxygen, flow rate and temperature by integrating more control units into the IAPSI microreactor. Furthermore, we will try to scale up the microreactor and work with other enzymes for the production of high performance functional materials.40–43
4 Conclusion
In conclusion, we proposed a one-step method to form the immobilized artificial PSI (IAPSI) in a microfluidic chip. This method is advantageous over the traditional three-step method in by having a simple process, promotion of the g-C3N4 and M by π–π stacking and a high coenzyme regeneration rate. The advantages of this method create the potential for artificial photosynthesis in the future.Acknowledgements
This work was supported by the National Science Foundation of China (no. 61377068), Research Grants Council (RGC) of Hong Kong (N_PolyU505/13, PolyU 5334/12E, and PolyU 152184/15E), and The Hong Kong Polytechnic University (G-YN07, G-YBBE, 4-BCAL, 1-ZVAW, 1-ZE14, A-PM21, 1-ZE27 and 1-ZVGH).References
- D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2009, 42, 1890–1898 CrossRef CAS PubMed.
- S. Berardi, S. Drouet, L. Francàs, C. Gimbert-Suriñach, M. Guttentag, C. Richmond, T. Stoll and A. Llobet, Chem. Soc. Rev., 2014, 43, 7501–7519 RSC.
- S. Takeuchi, S. Ruhman, T. Tsuneda, M. Chiba, T. Taketsugu and T. Tahara, Science, 2012, 338, 1321–1325 CrossRef PubMed.
- M. Mifsud, S. Gargiulo, S. Iborra, I. W. C. E. Arends, F. Hollmann and A. Corma, Nat. Commun., 2014, 5, 3145 Search PubMed.
- H. Zhou, J. Guo, P. Li, T. Fan, D. Zhang and J. Ye, Sci. Rep., 2013, 3, 1667 Search PubMed.
- X. H. Li, J. Zhang, X. Chen, A. Fischer, A. Thomas, M. Antonietti and X. Wang, Chem. Mater., 2011, 23, 4344–4348 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- Y. Maenaka, T. Suenobu and S. Fukuzumi, J. Am. Chem. Soc., 2012, 134, 367–374 CrossRef CAS PubMed.
- J. Liu, N. P. Wickramaratne, S. Z. Qiao and M. Jaroniec, Nat. Mater., 2015, 14, 763–774 CrossRef CAS PubMed.
- J. Liu, Q. Zhang, J. Yang, H. Ma, M. O. Tade, S. Wang and J. Liu, Chem. Commun., 2014, 50, 13971–13974 RSC.
- Z. Y. Zhao, J. Liu, M. Hahn, S. Qiao, A. P. J. Middelberg and L. He, RSC Adv., 2013, 3, 22008–22013 RSC.
- Y. Zheng, J. Liu, J. Liang, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2012, 5, 6717–6731 CAS.
- H. Zhou, X. Li, T. Fan, F. E. Osterloh, J. Ding, E. M. Sabio, D. Zhang and Q. Guo, Adv. Mater., 2010, 22, 951–956 CrossRef CAS PubMed.
- J. Barber, Chem. Soc. Rev., 2009, 38, 185–196 RSC.
- J. Liu and M. Antonietti, Energy Environ. Sci., 2013, 6, 1486–1493 CAS.
- F. Wang, W. G. Wang, X. J. Wang, H. Y. Wang, C. H. Tung and L. Z. Wu, Angew. Chem., Int. Ed., 2011, 50, 3193–3197 CrossRef CAS PubMed.
- J. Ryu, S. H. Lee, D. H. Nam and C. B. Park, Adv. Mater., 2011, 23, 1883–1888 CrossRef CAS PubMed.
- C. Luo, D. M. Guldi, H. Imahori, K. Tamaki and Y. Sakata, J. Am. Chem. Soc., 2000, 122, 6535–6551 CrossRef CAS.
- S. H. Lee, J. Ryu, D. H. Nam and C. B. Park, Chem. Commun., 2011, 47, 4643–4645 RSC.
- J. Huang, M. Antonietti and J. Liu, J. Mater. Chem. A, 2014, 2, 7686–7693 CAS.
- J. Liu, R. Cazelles, Z. P. Chen, H. Zhou, A. Galarneau and M. Antonietti, Phys. Chem. Chem. Phys., 2014, 16, 14699–14705 RSC.
- N. Wang, X. Zhang, Y. Wang, W. Yu and H. L. W. Chan, Lab Chip, 2014, 14, 1074–1082 RSC.
- T. M. Squires and S. R. Quake, Rev. Mod. Phys., 2005, 77, 977–1026 CrossRef CAS.
- G. M. Whitesides, Nature, 2006, 442, 368–373 CrossRef CAS PubMed.
- X. Huang, W. Hui, C. Hao, W. Yue, M. Yang, Y. Cui and Z. Wang, Small, 2014, 10, 758–765 CrossRef CAS PubMed.
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S. Lee, J. Zhong and Z. Kang, Science, 2015, 347, 970–974 CrossRef CAS PubMed.
- Z. Zhou, Y. Shen, Y. Li, A. Liu, S. Liu and Y. Zhang, ACS Nano, 2015, 9, 12480–12487 CrossRef CAS PubMed.
- J. Liu, H. Wang and M. Antonietti, Chem. Soc. Rev., 2016, 45, 2308–2326 RSC.
- J. Duan, S. Chen, M. Jaroniec and S. Z. Qiao, ACS Nano, 2015, 9, 931–940 CrossRef CAS PubMed.
- Z. Meng, X. Zhang and J. Qin, Nanoscale, 2013, 5, 4687–4690 RSC.
- M. Poizat, I. W. C. E. Arends and F. Hollmann, J. Mol. Catal. B: Enzym., 2010, 63, 149–156 CrossRef CAS.
- K. T. Oppelt, J. Gasiorowski, D. A. M. Egbe, J. P. Kollender, M. Himmelsbach, A. W. Hassel, N. S. Sariciftci and G. Knör, J. Am. Chem. Soc., 2014, 136, 12721–12729 CrossRef CAS PubMed.
- Y. Liu, L. Moevius, X. Xu, T. Qian, J. M. Yeomans and Z. Wang, Nat. Phys., 2014, 10, 515–519 CrossRef CAS.
- P. Niu, L. Zhang, G. Liu and H. M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- D. C. Duffy, J. C. McDonald, O. J. A. Schueller and G. M. Whitesides, Anal. Chem., 1998, 70, 4974–4984 CrossRef CAS PubMed.
- X. Huang, Y. Zhu, X. Zhang, Z. Bao, D. Y. Lei, W. Yu, J. Dai and Y. Wang, Sens. Actuators, B, 2016, 222, 106–111 CrossRef CAS.
- H. H. Zhao, H. Yu, X. Quan, S. Chen, Y. Zhang, H. H. Zhao and H. Wang, Appl. Catal., B, 2014, 152–153, 46–50 CrossRef CAS.
- F. Hollmann, B. Witholt and A. Schmid, J. Mol. Catal. B: Enzym., 2002, 19–20, 167–176 CrossRef CAS.
- J. S. Lee, S. H. Lee, J. H. Kim and C. B. Park, Lab Chip, 2011, 11, 2309–2311 RSC.
- X. Huang, W. Yue, D. Liu, J. Yue, J. Li, D. Sun, M. Yang and Z. Wang, Sci. Rep., 2016, 6, 23591 CrossRef CAS PubMed.
- S. Choudhury, J. O. Baeg, N. J. Park and R. K. Yadav, Angew. Chem., Int. Ed., 2012, 51, 11624–11628 CrossRef CAS PubMed.
- H. C. Lo and R. H. Fish, Angew. Chem., Int. Ed., 2002, 41, 478–481 CrossRef CAS PubMed.
- P. Könst, H. Merkens, S. Kara, S. Kochius, A. Vogel, R. Zuhse, D. Holtmann, I. W. C. E. Arends and F. Hollmann, Angew. Chem., Int. Ed., 2012, 51, 9914–9917 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21390a |
| This journal is © The Royal Society of Chemistry 2016 |