A new water-soluble heteronuclear PdII–AuI pincer complex as two-photon luminescent probe for biological Co2+ detection†
Abstract
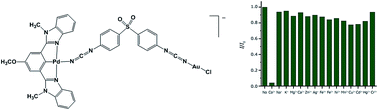
The heterodinuclear NCN pincer complex K[(L)PdII(DPS)AuICl], 1, (HL = 5-methoxy-1,3-bis(1-methyl-1H-benzo[d]imidazol-2-yl)benzene, DPSH2 = 4,4′-dicyanamidodiphenyl sulfone) with two-photon induced luminescent properties was synthesized and investigated for biological Co2+ detection. Importantly, 1 can selectively recognize Co2+ in aqueous media in the presence of other abundant cellular cations (such as Na+, K+, Mg2+, and Ca2+), trace metal ions in organisms (such as Zn2+, Ag+, Fe3+, Fe2+, Ni2+, Mn2+, and Cu2+), prevalent toxic metal ions in the environment (such as Cd2+, Hg2+, and Cr3+), and amino acids, with high sensitivity. The fluorescence response of 1-Co2+ with respect to pH change was studied and the resulting demonstrated fluorescence enhancement was observed in the pH range of 4–10. The water-soluble probe 1 was successfully applied in the visualizing of the site of Co2+ accumulation as well as estimating of trace amounts of Co2+ ions in live HeLa cells by two-photon microscopy.

 Please wait while we load your content...
Please wait while we load your content...