Synthesis and NMR analysis of 13C and 15N-labeled long-chain polyamines (LCPAs)†
Abstract
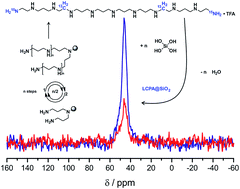
15N, 13C, or both isotope labels were introduced in selected positions of several LCPAs containing 5, 11, or 13 nitrogens. The number of transformations during chemical synthesis was minimized by coupling Fmoc-glycine-15N, Fmoc-glycine-1-13C, or Fmoc-β-alanine simultaneously at both ends of the growing oligoamide chain on resin-linked norspermidine. LCPAs containing 10–20 nitrogens are the main organic constituent of diatom biosilica. Preliminary NMR studies of bioinspired silica nanocomposites obtained from double-labeled LCPA 14 and 29Si-enriched orthosilicic acid identified the close spatial arrangement of 29Si and 13C nuclei.

 Please wait while we load your content...
Please wait while we load your content...