ZnO–graphene composites with high photocatalytic activities under visible light
Junli Xu*,
Ya Cui,
Yide Han,
Men Hao and
Xia Zhang
College of Science, Northeastern University, Shenyang, 110004, China. E-mail: jlxu@mail.neu.edu.cn; Fax: +86-24-83684533; Tel: +86-24-83684533
First published on 26th September 2016
Abstract
ZnO–graphene composites were prepared through a simple chemical corrosion process, and their photocatalytic performance on the degradation of methyl orange and water splitting was studied. The results showed that the prepared ZnO–graphene composites have a porous network structure, which is a benefit to the adsorption and mass transfer of dye and oxygen. The best degradation rate was 87% in 180 min under visible light irradiation with 8 mg ZnO–graphene photocatalyst and the rate of water splitting was up to 4.35 mmol g−1 h−1. The outstanding photocatalytic performance was ascribed to the porous network structure, narrow band energy and electronic properties of the composites. The presence of graphene can promote the separation of photogenerated electrons with holes, and repress charge carrier recombination, which results in an improvement of photocatalytic activity.
1. Introduction
The energy crisis and increasing environmental pollution are two serious problems currently. Photocatalysis has attracted intense attention because of its advantage in the complete mineralization of organic pollutants and hydrogen generation through water splitting. ZnO photocatalyst has attracted much attention because it is environmentally friendly, has a large excitation–binding energy and low price.1–4 However, most reported ZnO-based materials were wide band gap semiconductors5–7 and there exist some problems, such as low stability because of photo-corrosion effects during light irradiation, low quantum efficiency due to the fast recombination of photo-induced electron–hole pairs.8,9 Therefore, new preparation methods and composite materials have been explored to enhance their photocatalytic activities under visible light and retard the recombination of photo-generated electron–hole pairs of ZnO. It has been reported that some nano-ZnO show enhanced photocatalytic activity towards visible light photodegradation in recent years.10–14 For example, Zhang et al. reported that long ZnO nanorods showed a higher activity as compared to the shorter rods. The best degradation percentages after 80 min sodium lamp irradiation reached 99.3% in their report.14Hybridization is an important method to inhibit the recombination of photo-induced electrons and holes for ZnO. Graphene has unique electronic properties, large theoretical surface area and high transparency, which make it an excellent candidate for modifying a photocatalyst for enhanced photocatalytic performance.15 It was found that graphene and graphene oxide can significantly improve the separation efficiency of photo generated electron–hole pairs during a photocatalytic process.16–23 Furthermore, graphene can narrow the band gap of the semiconductor, which extends the range of efficient light absorption to the visible and near-IR range.24–27 In addition, graphene introduction is useful for enhanced absorptivity on the composite catalyst surface due to giant two-dimensional crystal lattice of graphene and a possibility of more π–π interaction between photocatalyst and organic compounds.28
ZnO–graphene hybrid can be synthesized by various routes, such as hydrothermal method,29,30 chemical vapor deposition31,32 and electrochemical deposition.33 These methods usually required high temperatures, vacuum systems, and rigorous experimental conditions. In addition, the product is obtained in low yields using these methods. Therefore, preparing a ZnO–graphene hybrid with good performance using single reaction step at low cost and temperature is highly desirable.
Herein, we report an easy and effective synthesis approach to prepare ZnO–graphene composites. During the synthesis process, graphene was first produced by electro-exfoliation of graphite in ionic liquids–water mixtures. The as-prepared graphene was dispersed in sulfuric acid under ultrasonication for 1.5 h. Finally, a ZnO–graphene hybrid was prepared by a simple chemical corrosion method using a well dispersed graphene sulfuric acid solution. The photocatalytic activities of the obtained ZnO–graphene composites, ZnO and graphene were investigated by evaluating in terms of the degradation of organic solution and water splitting under simulated sunlight irradiation. In addition, the effects of the structure and interface electronic interaction between ZnO and graphene on the photocatalytic activity were investigated systematically.
Compared to the reported preparation methods of ZnO–graphene composites, our synthesis approach is much simpler and rapider. More importantly, the ZnO and ZnO–graphene composites produced by our method show a multilayer porous structure, which is helpful to enhance the separation and transfer of photogenerated electron–hole pairs,34 and exhibit excellent photocatalytic performance under visible light irradiation.
2. Experimental
2.1 Preparation of ZnO/RGO nanocomposites
Graphene was first prepared by electrochemical exfoliation method in a solution of 1-butyl-3-methylimidazolium tetrafluoroborate (BMIMBF4) and deionized water at a DC potential of 10 V, as described in detail in ref. 35. The as-prepared graphene was dispersed in 10 mL 1.67 mol L−1 sulfuric acid with different contents under ultrasonication for 1.5 h. The mixed graphene–sulfuric acid solution was dropped onto a zinc plate (about 3 cm2 of geometric area, 0.5 mm thickness) and spread over the plate surface in a beaker. The eroded zinc plate was taken out after 10 min and rinsed with deionized water until pH 7 was achieved. It was then dried at room temperature in air for 24 h. According to the concentration of graphene in sulfuric acid, the products were labeled as ZnO (without graphene dispersed in sulfuric acid), ZG-1 (0.4 g L−1 graphene in sulfuric acid), ZG-2 (0.6 g L−1 graphene in sulfuric acid) and ZG-3 (0.8 g L−1 graphene in sulfuric acid).The formation of ZnO under our experiment conditions can be shown as follows:
| Zn + H2SO4 = ZnO + SO2 +H2O | (1) |
As ZnO dissolves in sulfuric acid forming zinc sulfate and water as shown in reaction (2), the process should not be held for a long time.
| ZnO + H2SO4 = ZnSO4 + H2O | (2) |
The possible processes for ZnO–graphene composite under this circumstance can be represented as follows:
| Zn + G(graphene) + H2SO4 → G(ZnO) + ZnO + ZnSO4 + SO2 + H2O | (3) |
The schematic of the fabrication of ZnO–graphene composite using sulfuric acid chemical corrosion process is presented in Fig. 1.
2.2 Characterization
The surface morphological features of the obtained graphene were investigated by transmission electron microscopy (TEM). TEM was carried out by employing a high resolution transmission electron microscope Tecnai G20 with an FEG source at 200 kV. The morphologies of the ZnO/RGO composites were characterized by a PANalytical B.V.'MPDDY2094 X-ray diffractometer (XRD) with Cu Kα radiation (λ = 1.5406 Å). Scanning electron microscopy (SEM) pictures and quantitative standard microanalyses were obtained using energy dispersive X-ray analysis (EDS) with a Zeiss ultra plus FESEM apparatus. Ultraviolet and visible diffusive reflectance spectra (UV-vis DRS) were taken on a UV-vis spectrometer (Perkin Elmer, Lambda 35); the scanned range being 200–800 nm against barium sulfate standard. Mott–Schottky measurements were performed in 0.5 mol L−1 NaSO4 solution using an electrochemistry workstation (CHI 660, China) with a three-electrode system. The obtained ZnO or ZnO–graphene material served as the working electrode. A platinum plate and a saturated Ag/AgCl were used as the counter electrode and reference electrode, respectively. The experiments were operated at room temperature.2.3 Photocatalytic activity tests
The photocatalytic activity tests of the resulting ZnO–graphene composites were investigated by evaluating in terms of the degradation of organic solution and water splitting under simulated sunlight irradiation. In the photodegradation experiments, methyl orange (MO) was chosen as the degradation material to examine the photo-degradation of the ZnO and ZnO–graphene composites. In each experiment, four pieces of samples were immersed horizontally into 100 mL MO solution (5.0 × 10−5 mol L−1). A 300 W xenon lamp with a power of 20.15 mW cm−2 (Beijing Science and Technology Co., Ltd Park Philae), was set inside a cylindrical reactor, and surrounded by a circulating water jacket to cool the lamp and minimize infrared radiation. A glass filter with 420 nm was employed to cut off UV light.The total weight of the catalyst was approximately 8 mg, which was determined by excluding the coating from Zn substrate by ultrasonic method. UV-vis spectrophotometer was used to determine the solution concentration of residual methyl orange at room temperature (TU-1900, Beijing Purkinje General Instrument Co., Ltd). The absorbance accuracy is ±0.002 Abs (0–0.5 Abs) and ±0.004 Abs (0.5–1.0 Abs).
For the water splitting tests, the photocatalytic performance of the samples were evaluated by H2 production from a mixed solution of 90 mL deionized water and 10 mL methanol as a hole scavenger under a 300 W xenon lamp with a power of 97.6 mW cm−2 (Beijing Science and Technology Co., Ltd Park Philae, emitting in the 200–1100 nm wave length range) in a quartz reactor with a top quartz window and a water cooling jacket. In a typical experiment, four pieces of samples (8 mg photocatalyst) were added to the bottom of the glass reactor without overlapping each sample. The glass reactor was degassed for 10 min by a vacuum pump and then irradiated under the 300 W Xe lamp. The distance between the reaction bottle and the light source was maintained at 10 cm and the light irradiation density was 97.6 mW cm−2. The hydrogen evolution was measured every 1 h with an online gas chromatograph (GC7900, TECHCOMP Ltd Co., China) equipped with a TCD detector.
3. Results and discussion
3.1 Characterization of ZnO and ZnO–graphene composite
Fig. 2 shows the typical morphological and microstructural analyses (from TEM and FESEM observations) of graphene, ZnO, and ZnO–graphene composites (ZG-2). Fig. 2(a) shows that the graphene sheets presented a crumpled surface. Porous ZnO and ZnO–graphene composites with a multilayered network structure were obtained with high uniformity. Moreover, the nets were constructed by nanosheets (Fig. 2(c) and (e)). There was no evident difference for the structure of ZnO after the introduction of graphene into ZnO.XRD was employed to investigate the phase of the as-prepared graphene and corrosion thin films, as shown in Fig. 3. Except for the substrate material, Zn, ZnO and ZnSO4 were also detected in the corrosion thin films. However, no characteristic peaks of graphene or graphite were observed for the ZnO–graphene composites, which may be due to the fact that the content of the graphene was low. As ZnSO4 is soluble in water, it could be washed out from the corrosion thin film during the rinsing process with deionized water after the corrosion process. The residual ZnSO4 in the corrosion thin films may be caused by adherence.
UV-vis diffused reflectance spectra of pristine ZnO, ZnO–graphene composites (ZG-1, ZG-2 and ZG-3) and graphene are shown in Fig. 4(a). From the curves of (αhν)2 versus hν derived from the UV-visible spectra (Fig. 4(b)), it is found that after the loading of graphene, the band gap energy decreased significantly compared to pristine ZnO and the extent of band gap energy depends greatly on the loading level. The pristine ZnO exhibits a gap energy of 2.67 eV, whereas ZnO–graphene composites exhibit a gap energy of about 2.37 eV (ZG-1), 1.77 eV (ZG-2) and 2.13 eV (ZG-3). It can be deduced that the presence of graphene has some influence on the electronic energy level of ZnO. Similar results were also reported by Hur et al. that hybrid RGO–ZnO exhibited a narrower band gap (2.16 eV) when compared to pure ZnO (3.06 eV).36 This phenomenon can be ascribed to the formation of Zn–O–C or Zn–C chemical bonds in the prepared composites.36–38
 | ||
| Fig. 4 (a) UV-vis diffused reflectance spectra of pristine ZnO and ZG samples; (b) plots of transformed Kubelka–Munk function versus the energy of light. | ||
The electronic properties of semiconductor can be investigated by Mott–Schottky (MS) analysis from capacitance measurements. The application of MS analysis is based on the assumption that the capacitance of the Helmholtz layer is much larger than the space-charge capacitance and the measured frequency is high enough so that the measured capacitance is equal to the space-charge capacitance.
 | (4) |
The MS plots are presented in Fig. 5 for pristine ZnO and ZnO–graphene composites (ZG-1, ZG-2 and ZG-3) samples. The slopes of the curves in the MS plots are positive for all the samples indicating the n-type nature of the samples. To estimate flat band potential (EFB), the linear part of the data was extrapolated to 1/Csc2 = 0. The EFB for ZnO, ZG-1, ZG-2 and ZG-3 samples estimated from MS plots were −0.898 V, −1.168 V, −1.448 V and −0.258 V (vs. Ag/AgCl), respectively, which equal to −3.70 eV, −3.53 eV, −3.25 eV and −4.44 eV, respectively. Combined with the results of UV-vis diffused reflectance spectra, the potential of the valence band of ZnO and ZnO–graphene composites can be calculated, and the results are summarized in Table 1.
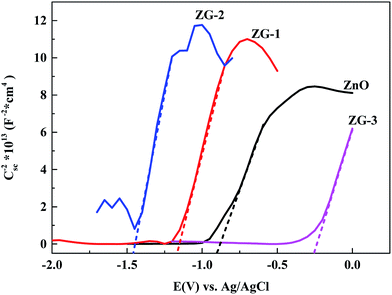 | ||
| Fig. 5 Mott–Schottky curves of the zinc oxide and zinc oxide/grapheme composites formed in sulfuric acid with different contents of graphene. | ||
| Semiconductor | Potential of the conduction band | Potential of the valence band | Band gap energy |
|---|---|---|---|
| ZnO | −3.70 eV | −6.47 eV | 2.67 eV |
| ZG-1 | −3.53 eV | −5.90 eV | 2.37 eV |
| ZG-2 | −3.25 eV | −5.02 eV | 1.77 eV |
| ZG-3 | −4.44 eV | −6.57 eV | 2.13 eV |
3.2 Photocatalytic properties
The photocatalytic activity of the ZnO and ZnO–graphene composites towards the degradation of MO under visible light irradiation was investigated, and the results of all the samples are shown in Fig. 6(a). It was observed that MB is very stable under visible light irradiation when graphene was used as the photocatalyst. No degradation occurs even after exposure for 3 h to the visible light. About 77% MO was photo-degraded in 3 h by pristine ZnO. Among the composites, the ZG-2 nanocomposite catalyst had the maximum degradation efficiency for the MO dye. About 83% and 87% MO was bleached in 3 h by ZG-1 and ZG-2. However, only about 73% MO was photo-degraded in 3 h by ZG-3.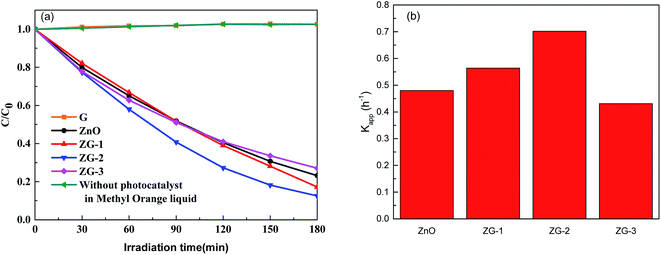 | ||
| Fig. 6 (a) Photo-degradation of MO by visible light irradiation in the presence of catalyst. (b) The apparent reaction rate constant of MO photodegradation in the presence of various catalysts. | ||
The photocatalytic efficiency of the materials was evaluated by calculating the apparent reaction rate constant for each photocatalyst according to the Langmuir–Hinshelwood pseudo-first order kinetics, as shown in eqn (5).39
 | (5) |
As shown in Fig. 6(b), the reaction constants of ZG-1 and ZG-2 composites are higher than that of ZnO, whereas the rate constants of ZG-3 are lower than that of ZnO. The highest rate constant obtained from ZG-2 (Kapp = 0.702 h−1) is about 1.5 times higher than that of ZnO (Kapp = 0.480 h−1).
Moreover, as shown in Table 1, the prepared ZnO and ZnO–graphene composites have a higher potential of conduction band than the potential of H+/H2, which indicate that these materials are suitable for the hydrogen reduction process by water splitting under visible light. The photocatalytic hydrogen evolution activities of all the prepared samples were investigated under visible light irradiation with an H2O–CH3OH solution, as presented in Fig. 7. Fig. 7(a) shows the hydrogen content calculated by the surface area of photocatalyst, whereas Fig. 7(b) presents the hydrogen content based on the calculation of the mass of the photocatalyst. The hydrogen evolution rate for every sample can be obtained from a linear fit of the data. As shown in Fig. 7, no hydrogen gas was produced when the experiment was performed using graphene as the photocatalyst and the optimal photocatalytic performance was obtained for GZ-2, reaching 4.35 mmol g−1 h−1.
 | ||
| Fig. 7 Activities of H2 evolution for ZnO and ZnO–graphene composites under Xe lamp irradiation for splitting water. | ||
3.3 Discussion on the photocatalytic mechanism
From Fig. 6 and 7, it is deduced that the introduction of graphene into ZnO to a center degree can favor the improvement of its photocatalytic activity. However, too much graphene introduction into ZnO can also decrease its photocatalytic activity. This can be attributed to two main reasons. First, as shown in Fig. 4(b), graphene introduction may narrow the band gap of ZnO, which widens the range of efficient light absorption. Secondly, graphene has been recognized as an electron collector and transporter to hinder electron/hole recombination and lengthen the electron/hole lifetime.15 As shown in Table 1, the potential of the conduction band and the valence band of ZnO is about −6.47 and −3.70 eV, whereas for graphene is about −4.42 eV.40 Therefore, it is thermodynamically favorable for direct electron transfer from the conduction band of ZnO to graphene, as shown in Fig. 8. Thus, the combination of ZnO with graphene can accelerate the division and restrain the recombination of photogenerated electron–hole pairs, resulting in improved photocatalytic activity. However, with further increase in the graphene content, the photocatalytic activity decreased, which is probably due to the fact that too much graphene would prevent light from reaching the surface of ZnO nanoparticles and block light adsorption because graphene is a black material with a 0 eV bandgap and it will absorb a large amount of incident light41,42 and thus reduce the generation of electrons and holes.4. Conclusions
ZnO and ZnO–graphene composites were synthesized successfully by chemical corrosion method. The synthesized ZnO and ZnO–graphene composites showed good photocatalytic activity towards the degradation of MO under visible-light irradiation and hydrogen production in the water–methanol system under simulated solar light irradiation. The photocatalytic activity is affected by the graphene content in ZnO. About 87% MO was bleached in 3 h by 8 mg ZnO–graphene composites, and its hydrogen production rate reached 4.35 mmol g−1 h−1. The improved photocatalytic activity was attributed to the presence of graphene as a supporting material, which enhances the absorption capacity and suppresses the recombination of photo-generated charge carriers.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 51574071 and No. 21501023).References
- M. M. Hossain, B. C. Ku and J. R. Hahn, Appl. Surf. Sci., 2015, 354, 55 CrossRef CAS.
- Y. Yang, L. L. Ren, C. Zhang, S. Huang and T. X. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 2779 CAS.
- H. B. Fu, T. G. Xu, S. B. Zhu and Y. F. Zhu, Environ. Sci. Technol., 2008, 42, 8064 CrossRef CAS PubMed.
- X. Wu, L. Wen, K. Lv, K. Deng, D. Tang, H. Ye, D. Du, S. Liu and M. Li, Appl. Surf. Sci., 2015, 358, 130 CrossRef CAS.
- H. Yu, H. Ming, J. Gong, H. Li, H. Huang, K. Pan, Y. Liu, Z. Kang, J. Wei and D. Wang, Bull. Mater. Sci., 2013, 36, 367 CrossRef CAS.
- M. Nirmala, M. G. Nair, K. Rekha, A. Anukaliani, S. K. Samdarshi and R. G. Nair, Afr. J. Basic Appl. Sci., 2010, 2, 161 Search PubMed.
- C. C. Lin and Y. Y. Li, Mater. Chem. Phys., 2009, 113, 334 CrossRef CAS.
- C. Ren, B. Yang, M. Wu, J. Xu, Z. Fu, Y. Lv, T. Guo, Y. Zhao and C. Zhu, J. Hazard. Mater., 2010, 182, 123 CrossRef CAS PubMed.
- H. Moussa, E. Girot, K. Mozet, H. Alem, G. Medjahdi and R. Schneider, Appl. Catal., B, 2016, 185, 11 CrossRef CAS.
- F. Peng, S. H. Chen, L. Zhang, H. J. Wang and Z. Y. Xie, Acta Phys.-Chim. Sin., 2005, 21, 944 CAS.
- M. A. Habib, M. Muslim, M. T. Shahadat, M. N. Islam, I. M. I. Ismail, T. S. A. Islam and A. J. Mahmood, J. Nanostruct. Chem., 2013, 3, 70 CrossRef.
- S. Kuriakose, N. Bhardwaj, J. Singh, B. Satpati and S. Mohapatra, Beilstein J. Nanotechnol., 2013, 4, 763 CrossRef CAS PubMed.
- A. Sapkota, A. J. Anceno, S. Baruah, O. V. Shipin and J. Dutta, Nanotechnology, 2011, 22, 215703 CrossRef PubMed.
- X. Zhang, J. Qin, Y. Xue, P. Yu, B. Zhang, L. Wang and R. Liu, Nat., Sci. Prog., 2014, 4, 4596 Search PubMed.
- H. Sun and S. Wang, Energy Fuels, 2014, 28, 22 CrossRef CAS.
- N. Zhang, Y. Zhang and Y. J. Xu, Nanoscale, 2012, 4, 5792 RSC.
- Y. Sun, Q. Wu and G. Shi, Energy Environ. Sci., 2011, 4, 1113 CAS.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782 RSC.
- B. Li, T. Liu, Y. Wang and Z. Wang, J. Colloid Interface Sci., 2012, 377, 114 CrossRef CAS PubMed.
- K. Wang, J. Xu and X. Wang, Appl. Surf. Sci., 2016, 360, 270 CrossRef.
- B. J. Li and H. Q. Cao, Mater. Chem., 2011, 21, 3346 RSC.
- J. Qin, X. Zhang, Y. Xue, N. Kittiwattanothai, P. Kongsittikul, N. Rodthongkum, S. Limpanart, M. Ma and R. Liu, Appl. Surf. Sci., 2014, 321, 226 CrossRef CAS.
- S. An, B. N. Joshi, M. W. Lee, N. Y. Kim and S. S. Yoon, Appl. Surf. Sci., 2014, 294, 24 CrossRef CAS.
- P. Chen, T. Y. Xiao, H. H. Li, J. J. Yang, Z. Wang, H. B. Yao and S. H. Yu, ACS Nano, 2011, 6, 712 CrossRef PubMed.
- N. Zhang, Y. Zhang, M. Q. Yang, Z. R. Tang and Y. J. Xu, J. Catal., 2013, 299, 210 CrossRef CAS.
- Y. Liu, Y. Hu, M. Zhou, H. Qian and X. Hu, Appl. Catal., B, 2012, 125, 425 CrossRef CAS.
- Q. Zhang, C. Tian, A. Wu, T. Tan, L. Sun, L. Wang and H. Fu, J. Mater. Chem., 2012, 22, 11778 RSC.
- B. Sun, Y. Dong, Z. Jin, Q. Wang and Z. Lei, Ceram. Int., 2016, 42, 7632 CrossRef.
- D. Kale and P. Thakur, J. Porous Mater., 2015, 22, 797 CrossRef CAS.
- S. Liu, H. Sun, A. Suvorova and S. Wang, Chem. Eng. J., 2011, 229, 533 CrossRef.
- R. Cai, J. G. Wu, L. Sun, Y. J. Liu, T. Fang, S. Zhu, S. Y. Li, Y. Wang, L. F. Guo, C. E. Zhao and A. Wei, Mater. Des., 2016, 90, 839 CrossRef CAS.
- R. K. Biroju, N. Tilak, G. Rajender, S. Dhara and P. K. Giri, Nanotechnology, 2015, 26, 145601 CrossRef PubMed.
- A. A. Ashkarran and B. Mohammadi, Appl. Surf. Sci., 2015, 342, 112 CrossRef CAS.
- M. Zhang, J. Xu, R. Zong and Y. Zhu, Appl. Catal., B, 2014, 147, 229 CrossRef CAS.
- J. L. Xu, Z. N. Shi, X. Zhang and G. M. Haarberg, Mater. Res. Express, 2014, 1, 045606 CrossRef.
- H. N. Tien, V. H. Luan, L. T. Hoa, N. T. Khoa, S. H. Hahn, J. S. Chung, E. W. Shin and S. H. Hur, Chem. Eng. J., 2013, 229, 126 CrossRef CAS.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2009, 4, 380 CrossRef PubMed.
- L. Tian, L. Ye, K. Deng and L. Zan, J. Solid State Chem., 2011, 184, 1465 CrossRef CAS.
- Y. Li, X. Li, J. Li and J. Yin, Water Res., 2006, 40, 1119 CrossRef CAS PubMed.
- A. Wei, L. Xiong, L. Sun, Y. Liu, W. Li, W. Lai, X. Liu, L. Wang, W. Huang and X. Dong, Mater. Res. Bull., 2013, 48, 2855 CrossRef CAS.
- H. Sun, G. Zhou, Y. Wang, A. Suvorova and S. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 16745 CAS.
- Y. Bu, Z. Chen, W. Li and B. Hou, ACS Appl. Mater. Interfaces, 2013, 5, 12361 CAS.
| This journal is © The Royal Society of Chemistry 2016 |




