Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On the stability of surfactant-stabilised few-layer black phosphorus in aqueous media†
Jack R.
Brent
a,
Ashok K.
Ganguli
bc,
Vinod
Kumar
b,
David J.
Lewis
*a,
Paul D.
McNaughter
 d,
Paul
O'Brien
*ad,
Priyanka
Sabherwal
b and
Aleksander A.
Tedstone
d
d,
Paul
O'Brien
*ad,
Priyanka
Sabherwal
b and
Aleksander A.
Tedstone
d
aSchool of Materials, University of Manchester, Oxford Road, M13 9PL, UK. E-mail: david.lewis-4@manchester.ac.uk; paul.o'brien@manchester.ac.uk
bInstitute of Nano Science & Technology (INST), Habitat Centre, Phase-10, Sec-64, Mohali-160062, Punjab, India
cDepartment of Chemistry, Indian Institute of Technology Delhi, Hauz Khas, New Delhi-110016, India
dSchool of Chemistry, University of Manchester, Oxford Road, M13 9PL, UK
First published on 7th September 2016
Abstract
Surfactant-assisted exfoliation routes to few-layer black phosphorus in aqueous media have recently been reported. The stability of such few-layer black phosphorus has been studied using a range of spectroscopic techniques. The material is meta-stable in aqueous media, degrading over time mainly to phosphoric acids.
Graphene and other two-dimensional (2D) materials have revolutionised materials science.1 The properties displayed by 2D materials are markedly different to their bulk counterparts.2 For instance, graphene shows ballistic carrier mobility and is a superb conductor of electricity,3,4 whilst monolayer molybdenum disulfide becomes a direct bandgap semiconductor with strong photoluminescence.5 Hence, 2D materials have attracted great interest over the past decade and have the potential to revolutionise a range of fields.6
2D materials may be synthesised in several different ways. Micromechanical pinacoidal cleavage of bulk crystals (the Scotch Tape method), made popular by Geim and co-workers,7 has been the most popular of all the processing techniques; but is strictly limited to demonstrative studies. More useful perhaps is the large scale growth of polycrystalline wafers of 2D materials by chemical vapour deposition.8 One of the simplest ways that these materials can be produced, and additionally in solution, is by ultrasonic cavitation, which has been applied to most crystals that show basal cleavage.9–13 Shear exfoliation in surfactants has recently been reported, and can potentially be carried out on scale.14
Recently, there has been interest in elemental 2D synthetic allotropes which can show various properties complimentary to graphene. Silicene (2D silicon),15 borophene (2D boron),16 germanene (2D germanium)17 and stanene (2D tin)18 have all either been reported or predicted.19 One such elemental analogue, phosphorene (2D black phosphorus), has attracted attention due to its high p-type carrier mobility and its layer-dependent band gap that spans the range between graphene (negligible band gap) and 2D MoS2 (ca. 1.5 eV).20 A range of functional devices based on this material have been produced including transistors.21 Phosphorene is produced by exfoliation of the layered black phosphorus22 allotrope. Synthetic routes to the material include micromechanical cleavage20 and solution phase exfoliation.23–25 CVD routes to this material remain elusive.
Questions remain regarding the stability of phosphorene, or few-layer black phosphorus (FL-BP), that may stymie its eventual exploitation. The major problem is that the material is reactive to oxygen-containing species due to the strength of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Studies have shown that water in particular causes degradation of FL-BP in solution, as well as in exposed flakes. The solution-exfoliated material is more stable than micromechanically exfoliated material. Strategies to prevent chemical degradation of FL-BP have included coating with oxides,26 entombment within other 2D materials by vertical stacking of flakes,27 and, in the case of solution exfoliated material, physical barriers to atmospheric ingress28 and the use of sterically hindered solvents such as cyclohexyl-2-pyrrolidone (CHP).25
O bond. Studies have shown that water in particular causes degradation of FL-BP in solution, as well as in exposed flakes. The solution-exfoliated material is more stable than micromechanically exfoliated material. Strategies to prevent chemical degradation of FL-BP have included coating with oxides,26 entombment within other 2D materials by vertical stacking of flakes,27 and, in the case of solution exfoliated material, physical barriers to atmospheric ingress28 and the use of sterically hindered solvents such as cyclohexyl-2-pyrrolidone (CHP).25
Very recently, Hersam and co-workers introduced the concept of surfactant-assisted ultrasonic exfoliation to produce dispersions of FL-BP in aqueous media.29 In this study, the FL-BP sols were produced by ultrasonication of layered bulk black phosphorus in degassed water containing 2% w/v sodium dodecyl sulfate (SDS) surfactant. This presents an elegant solution-based strategy to protect FL-BP from degradation in theory. The authors claim that the solutions are stable, yet no stability study over time was presented. Ultimately the removal of organic solvents from processing is a well-principled aim in terms of scale up due to the cost of NMP and for environmental concerns and thus merits investigation. In this paper we present a multi-spectroscopic temporal stability study of surfactant-exfoliated FL-BP sols in aqueous media.
We exfoliated FL-BP using 1% w/v Triton X-100 (TX-100, C14H22O(C2H4O)n where n = 9–10) surfactant in water. In a sealed vial, the degassed surfactant solution (15 mL) was added to bulk BP (100 mg) and the vial was flushed with argon, closed and sealed with Parafilm®. Suspensions were sonicated at 298 K in an Elmasonic P 70 H bench-top ultrasonic bath (820 W across four horns) operating at 37 kHz and 30% power. After 36 h the dispersions were centrifuged at 1500 rpm for 45 min and the supernatant removed. Further 10 mL of surfactant solution was added to the pellet of FL-BP which was sonicated again for 12 h under the same conditions. The dispersion was centrifuged again and the supernatant was added to the previously collected dispersion. Our developed procedure, reported previously, produces FL-BP of sub-20 nm thickness and ca. 100–200 nm in side.30
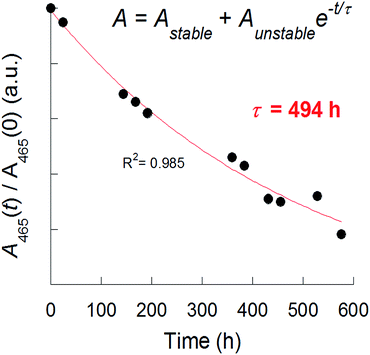
The stability of the nanosheets, was assessed by the optical absorbance at 465 nm over time. The degradation of the flakes was fitted to an empirical decay function used previously by Coleman and co-workers for FL-BP nanosheets:25
At/A0 = Astable + Aunstable![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−t/τ e−t/τ | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−t/τ), where τ is observed decay constant of the nanosheets. Thus by recording the UV-Vis absorbance at 465 nm over 600 h, it was found that the observed decay constant of the exfoliated nanosheets (in 1% w/v aqueous Triton X-100 solution) was τ = 494 h (Fig. 1). The total percentage degradation of the sheets in solution was around 80% over this time, estimated from the total decrease in absorbance of the sol. FL-BP exfoliated in N-cyclohexyl-2-pyrrolidone (CHP) have decay constants in the range 115 to 350 h, and the total degradation of CHP-exfoliated BP is reported to be 25%, and is believed to be limited by the amount of water in the organic solvent. It is clear from the observed decay constant we measure here then that the surfactant plays a crucial role in slowing down the degradation process, protecting the surface and edges of the nanosheets from water, possibly by micelle formation. We postulate that the Triton X-100 head group, consisting of tBu(CH2)2Ph-R which is highly non-polar, is able to form an effective surface-bound layer protecting the BP from degradation by the surrounding water. The polar polyethylene oxide chains of the surfactant extend into the aqueous media. Lewis et al. have demonstrated previously that surfactants are useful for both the dispersion of main group oxide nanoparticles, as well as enhancement of the photoluminescence emission of surface-bound luminophores that are quenched by water and oxygen.31
e−t/τ), where τ is observed decay constant of the nanosheets. Thus by recording the UV-Vis absorbance at 465 nm over 600 h, it was found that the observed decay constant of the exfoliated nanosheets (in 1% w/v aqueous Triton X-100 solution) was τ = 494 h (Fig. 1). The total percentage degradation of the sheets in solution was around 80% over this time, estimated from the total decrease in absorbance of the sol. FL-BP exfoliated in N-cyclohexyl-2-pyrrolidone (CHP) have decay constants in the range 115 to 350 h, and the total degradation of CHP-exfoliated BP is reported to be 25%, and is believed to be limited by the amount of water in the organic solvent. It is clear from the observed decay constant we measure here then that the surfactant plays a crucial role in slowing down the degradation process, protecting the surface and edges of the nanosheets from water, possibly by micelle formation. We postulate that the Triton X-100 head group, consisting of tBu(CH2)2Ph-R which is highly non-polar, is able to form an effective surface-bound layer protecting the BP from degradation by the surrounding water. The polar polyethylene oxide chains of the surfactant extend into the aqueous media. Lewis et al. have demonstrated previously that surfactants are useful for both the dispersion of main group oxide nanoparticles, as well as enhancement of the photoluminescence emission of surface-bound luminophores that are quenched by water and oxygen.31
We further probed the stability of the BP nanosheets in aqueous media by considering solutions with the nanosheets removed by centrifugation followed by a polish with membrane ultrafiltration to leave clear, non-hazy, mother liquors. Inductively coupled plasma optical emission spectroscopy (ICP-OES) on these mother liquors revealed that the decrease in the absorption at 465 nm observed over time i.e. the degradation of FL-BP nanosheets, is accompanied by the release of phosphorus species into solution (Fig. 2). The amount of phosphorus detected by ICP-OES rises to a plateau at around in around 500 h, suggesting that there is a critical point at which the nanosheets cannot degrade any further, presumably where a critical micelle concentration is reached. There is no possibility that these phosphorus species remain bound to the surface of the FL-BP sheets as these are removed by the centrifugation–ultrafiltration procedure we employ and hence we are confident that we are looking solely at breakdown products that have been released into solution.
 | ||
| Fig. 2 ICP-OES time study of the absolute concentration of phosphorus released into solution from degradation of the FL-BP nanosheets in 1% w/v aqueous Triton X-100, showing a plateau-like profile. | ||
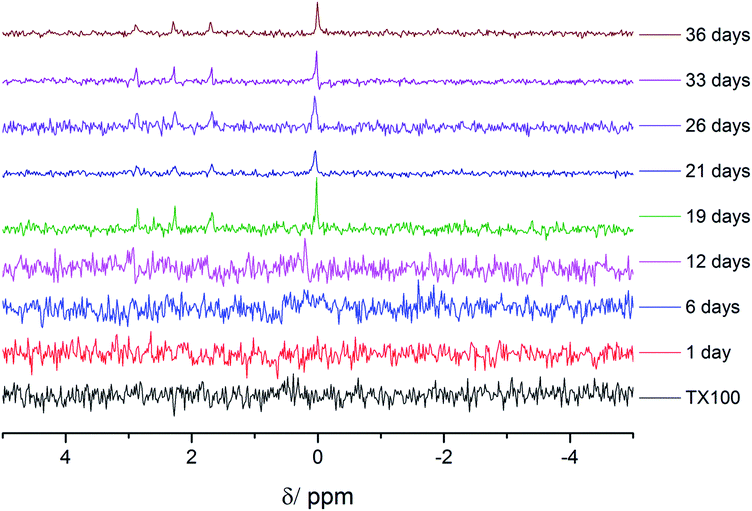
The chemical identity of the species released was probed by 31P nuclear magnetic resonance (NMR) spectroscopy using H3PO4 as a standard at 0 ppm (Fig. 3). The peaks which appear at ca. 1–3 ppm were assigned to orthophosphate, (PO43−). Hence, we can confidently state that the sheets degrade to phosphates in solution from hydrolytic mechanisms, and that the nanosheets are meta-stable when dispersed in aqueous media and thus have a ‘shelf life’.
In conclusion, we have shown that FL-BP nanosheets can be stabilised in aqueous media by the use of surfactants, however, there is some relatively slow degradation of the freshly formed material to form free POx phosphate species. Care should hence be taken when using FL-BP sols to account for the possibility of this degradation over time. It seems however, that a more stable form can be reached in which further degradation is limited and in this sense the sols appear to be meta-stable. This will be important when, for instance, considering the biological applications of phosphorene, some of which have recently been reported.32
Acknowledgements
P. O. B., P. D. M. and J. R. B. would like to thank the Parker family and EPSRC (EP/K009710/1) for funding. V. K. and P. S. acknowledge INST PDF, CSIR-JRF, and DST, Govt. of India for funding. A. K. G. thanks DST, Govt. of India for financial support to INST, Mohali, India. Some of the equipment used in this study were provided by EPSRC (Core Capability in Chemistry, EPSRC grant number EP/K039547/1). Additional data associated with this paper is available from the University of Manchester's Pure repository. The authors would also like to thank Paul Lythgoe (University of Manchester) for running ICP-OES and Dr Ralph Adams (University of Manchester) for guidance with NMR measurements.Notes and references
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–200 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- P. Miro, M. Audiffred and T. Heine, Chem. Soc. Rev., 2014, 43, 6537–6554 RSC.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morosov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- A. Reina, X. T. Jia, J. Ho, D. Nezich, H. B. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Nano Lett., 2009, 9, 30–35 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. S. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- A. O'Neill, U. Khan and J. N. Coleman, Chem. Mater., 2012, 24, 2414–2421 CrossRef.
- N. Al-Dulaimi, E. A. Lewis, D. J. Lewis, S. K. Howell, S. J. Haigh and P. O'Brien, Chem. Commun., 2016, 52, 7878–7881 RSC.
- J. R. Brent, D. J. Lewis, T. Lorenz, E. A. Lewis, N. Savjani, S. J. Haigh, G. Seifert, B. Derby and P. O'Brien, J. Am. Chem. Soc., 2015, 137, 12689–12696 CrossRef CAS PubMed.
- K. R. Paton, E. Varrla, C. Backes, R. J. Smith, U. Khan, A. O'Neill, C. Boland, M. Lotya, O. M. Istrate, P. King, T. Higgins, S. Barwich, P. May, P. Puczkarski, I. Ahmed, M. Moebius, H. Pettersson, E. Long, J. Coelho, S. E. O'Brien, E. K. McGuire, B. M. Sanchez, G. S. Duesberg, N. McEvoy, T. J. Pennycook, C. Downing, A. Crossley, V. Nicolosi and J. N. Coleman, Nat. Mater., 2014, 13, 624–630 CrossRef CAS PubMed.
- L. Tao, E. Cinquanta, D. Chiappe, C. Grazianetti, M. Fanciulli, M. Dubey, A. Molle and D. Akinwande, Nat. Nanotechnol., 2015, 10, 227–231 CrossRef CAS PubMed.
- A. J. Mannix, X.-F. Zhou, B. Kiraly, J. D. Wood, D. Alducin, B. D. Myers, X. Liu, B. L. Fisher, U. Santiago, J. R. Guest, M. J. Yacaman, A. Ponce, A. R. Oganov, M. C. Hersam and N. P. Guisinger, Science, 2015, 350, 1513–1516 CrossRef CAS PubMed.
- M. E. Dávila, L. Xian, S. Cahangirov, A. Rubio and G. L. Lay, New J. Phys., 2014, 16, 095002 CrossRef.
- F.-f. Zhu, W.-j. Chen, Y. Xu, C.-l. Gao, D.-d. Guan, C.-h. Liu, D. Qian, S.-C. Zhang and J.-f. Jia, Nat. Mater., 2015, 14, 1020–1025 CrossRef CAS PubMed.
- S. Balendhran, S. Walia, H. Nili, S. Sriram and M. Bhaskaran, Small, 2015, 11, 640–652 CrossRef CAS PubMed.
- H. Liu, A. T. Neal, Z. Zhu, Z. Luo, X. Xu, D. Tománek and P. D. Ye, ACS Nano, 2014, 8, 4033–4041 CrossRef CAS PubMed.
- L. Li, Y. Yu, G. J. Ye, Q. Ge, X. Ou, H. Wu, D. Feng, X. H. Chen and Y. Zhang, Nat. Nanotechnol., 2014, 9, 372–377 CrossRef CAS PubMed.
- R. Hultgren, N. S. Gingrich and B. E. Warren, J. Chem. Phys., 1935, 3, 351–355 CrossRef CAS.
- J. R. Brent, N. Savjani, E. A. Lewis, S. J. Haigh, D. J. Lewis and P. O'Brien, Chem. Commun., 2014, 50, 13338–13341 RSC.
- P. Yasaei, B. Kumar, T. Foroozan, C. Wang, M. Asadi, D. Tuschel, J. E. Indacochea, R. F. Klie and A. Salehi-Khojin, Adv. Mater., 2015, 27, 1887–1892 CrossRef CAS PubMed.
- D. Hanlon, C. Backes, E. Doherty, C. S. Cucinotta, N. C. Berner, C. Boland, K. Lee, A. Harvey, P. Lynch, Z. Gholamvand, S. Zhang, K. Wang, G. Moynihan, A. Pokle, Q. M. Ramasse, N. McEvoy, W. J. Blau, J. Wang, G. Abellan, F. Hauke, A. Hirsch, S. Sanvito, D. D. O'Regan, G. S. Duesberg, V. Nicolosi and J. N. Coleman, Nat. Commun., 2015, 6, 8563 CrossRef CAS PubMed.
- J. D. Wood, S. A. Wells, D. Jariwala, K.-S. Chen, E. Cho, V. K. Sangwan, X. Liu, L. J. Lauhon, T. J. Marks and M. C. Hersam, Nano Lett., 2014, 14, 6964–6970 CrossRef CAS PubMed.
- Y. Cao, A. Mishchenko, G. L. Yu, E. Khestanova, A. P. Rooney, E. Prestat, A. V. Kretinin, P. Blake, M. B. Shalom, C. Woods, J. Chapman, G. Balakrishnan, I. V. Grigorieva, K. S. Novoselov, B. A. Piot, M. Potemski, K. Watanabe, T. Taniguchi, S. J. Haigh, A. K. Geim and R. V. Gorbachev, Nano Lett., 2015, 15, 4914–4921 CrossRef CAS PubMed.
- J. Kang, J. D. Wood, S. A. Wells, J. Lee, X. Liu, K. Chen and M. C. Hersam, ACS Nano, 2015, 9, 3596–3604 CrossRef CAS PubMed.
- J. Kang, S. A. Wells, J. D. Wood, J.-H. Lee, X. Liu, C. R. Ryder, J. Zhu, J. R. Guest, C. A. Husko and M. C. Hersam, Proc. Natl. Acad. Sci. U. S. A., 2016 DOI:10.1073/pnas.1602215113.
- V. Kumar, J. R. Brent, M. Shorie, H. Kaur, G. Chadha, A. G. Thomas, E. A. Lewis, A. P. Rooney, L. Nguyen, X. L. Zhong, M. G. Burke, S. J. Haigh, A. S. Walton, P. D. McNaughter, A. A. Tedstone, N. Savjani, C. A. Muryn, P. O'Brien, A. K. Ganguli, D. J. Lewis and P. Sabherwal, ACS Appl. Mater. Interfaces, 2016, 8, 22860–22868 CAS.
- D. J. Lewis, V. Dore, N. J. Rogers, T. K. Mole, G. B. Nash, P. Angeli and Z. Pikramenou, Langmuir, 2013, 29, 14701–14708 CrossRef CAS PubMed.
- H. Wang, X. Yang, W. Shao, S. Chen, J. Xie, X. Zhang, J. Wang and Y. Xie, J. Am. Chem. Soc., 2015, 137, 11376–11382 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, instrumentation and detailed exfoliation procedure. See DOI: 10.1039/c6ra21296d |
| This journal is © The Royal Society of Chemistry 2016 |