SnS2 nanoflakes for efficient humidity and alcohol sensing at room temperature†
Lakshmi Deepika Bharatulaa,
Manisha B. Erandea,
Imtiaz S. Mullaa,
Chandra Sekhar Routb and
Dattatray J. Late*a
aPhysical and Material Chemistry Division, CSIR – National Chemical Laboratory, Dr. Homi Bhabha Road, Pune, 411008, Maharashtra, India. E-mail: datta099@gmail.com; dj.late@ncl.res.in
bSchool of Basic Sciences, Indian Institute of Technology, Bhubaneswar 751013, India
First published on 28th October 2016
Abstract
We report a one step facile hydrothermal synthesis of layered SnS2 nanoflakes. The as-synthesized nanosheets are characterized using X-ray diffraction, Raman spectroscopy and Transmission Electron Microscopy (TEM). The humidity sensing behavior of SnS2 nanoflake sensor device were investigated in the range of 11–97% of relative humidity (RH) at room temperature. The response time of ∼85 s and recovery time of ∼6 s were observed for the SnS2 nanoflake based humidity sensor. A maximum sensitivity of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300% is recorded. We also investigate the SnS2 nanoflake based alcohol sensing properties towards methanol, ethanol and iso-propyl alcohol. An exclusive selectivity towards methanol with a response of 1580 is shown as compared to other analytes. The response time of ∼67 s and recovery time of just 5 s were observed for the SnS2 nanoflake based methanol sensor.
300% is recorded. We also investigate the SnS2 nanoflake based alcohol sensing properties towards methanol, ethanol and iso-propyl alcohol. An exclusive selectivity towards methanol with a response of 1580 is shown as compared to other analytes. The response time of ∼67 s and recovery time of just 5 s were observed for the SnS2 nanoflake based methanol sensor.
Introduction
Graphene has changed nanotechnology especially nanoelectronics since its invention.1–3 Its two dimensional (2D) hexagonal sheet structure has been widely exploited by many researchers for various applications like photocatalysis,4,5 optoelectronics,6,7 supercapacitors,8 sensors3,9,10 etc. But its semi-metallic nature has hindered its use in nanoelectronics and optoelectronic device applications. Recently, graphene analogous materials such as metal dichalcogenides like MoS2,11–14 WS2,15,16 MoSe2,17,18 WSe2,19–21 SnSe2,22 MoTe2,23 WTe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and other 2D inorganic materials such as black phosphorus25 and TiS3,26 etc. are widely studied to fill this void due to their extraordinary properties and performance in nanoelectronics devices as well as other applications such as optoelectronics, nanoelectronics, spintronics, catalysis etc.11–26
24 and other 2D inorganic materials such as black phosphorus25 and TiS3,26 etc. are widely studied to fill this void due to their extraordinary properties and performance in nanoelectronics devices as well as other applications such as optoelectronics, nanoelectronics, spintronics, catalysis etc.11–26
The tin disulphide (SnS2) is a layered material crystallized similar to CdI2 type where the tin cations (filling the octahedral sites of the alternating layers) and sulphide anions form hexagonally closed packed structures. The layered SnS2 is being extensively investigated due to its non-toxicity27,28 and high stability29,30 etc. It has been reported as an efficient material in various applications like Li-ion battery,31 NO2 sensing,32 glucose sensing,33 solar cells,34 field emission35 and enzyme biosensing applications36–38 etc.
An important property which is needed for the vapor sensing is the number of gas adsorption sites available for the sensing. This mainly depends on the morphology of the material. In the past different morphologies of SnS2 like nanoparticles,39 nanotubes,39 nanoflowers35 and nanowires40 were reported. Earlier reports on SnS2 nanosheets synthesis by Gao et al.41 and Zhuo et al.42 employed ammonia solution and NaOH to control the surface morphology and porosity. We have reported earlier nanosheets of SnS2 by direct mixing of precursors in an autoclave, which leds to formation of atomically thin nanosheets.35 High surface area is very crucial for sensing applications as it plays an important role.
Humidity hindrance is a huge problem in the industrial sector. Similarly, low concentration alcohol detection is a challenge in industrial plant. Hence, it is necessary to develop a sensor with high sensitivity and faster response and recovery time for enhanced sensing in the fields of environmental control, biomedicine, chemical industries etc.43,44 Recently, 2D materials such as black phosphorus,43,45 MoS2,46 WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 etc. have been reported for alcohol vapor sensing. But most of them are synthesized using mechanical exfoliation or chemical vapor deposition techniques which are not economically feasible. Hence, we employed simple hydrothermal synthesis method for economically viable and for more yieldable synthesis. Also, the sensing measurements were carried out at room temperature and in absence of vacuum to simulate the industrial environment.
47 etc. have been reported for alcohol vapor sensing. But most of them are synthesized using mechanical exfoliation or chemical vapor deposition techniques which are not economically feasible. Hence, we employed simple hydrothermal synthesis method for economically viable and for more yieldable synthesis. Also, the sensing measurements were carried out at room temperature and in absence of vacuum to simulate the industrial environment.
Experimental
Synthesis of SnS2 nanoflakes
In a typical synthesis, 0.7 g of SnCl4·5H2O was taken in 30 ml distilled (DI) water. This was stirred with the aid of magnetic stirrer till a transparent solution was obtained. Then, 1.2 g thioacetamide (TAA) was added to this solution and stirred till a yellowish solution was obtained. Meanwhile, 1 M of NaOH solution was prepared. The solution was then transferred to a 50 ml capacity Teflon lined autoclave and subjected to hydrothermal synthesis at 200 °C for 24 h in furnace. The furnace was allowed to cool naturally at room temperature. The resultant product was then centrifuged for 50 min at 7000 rpm using DI water and ethanol followed by drying at 90 °C in vacuum oven.Material characterizations
The structural identification was done by X-ray diffraction using a PANalytical X'pert Pro duel goniometer diffractometer equipped with filter for CuKα irradiation at λ = 1.5406 Å. The Raman spectra was recorded using Renishaw microscope at a wavelength of 632 nm. The morphology was investigated with the help of transmission electron microscope (TECNAI G2-20-TWIN, FEI, The Netherlands) operating at 200 keV.Humidity sensing test
The saturated salt solutions of various salts for achieving relative humidity environments were prepared in close vessel as reported earlier.25,48,49Alcohol sensing test
To measure alcohol sensing characteristics, argon gas was bubbled at a constant flow rate of 50 sccm through a gas bubbler containing alcohol to get vapors with desired concentration into the test chamber. We investigated for two concentrations ∼40 ppm and ∼150 ppm.Device fabrication
For the device fabrication, we used a 1 cm × 1 cm ITO substrate. A channel was made on this substrate by creating a scratch using a cutter so that the two sides of the channel act as source and drain. A channel width of 500 μm was noted. A thick paste of SnS2 nanoflake was then drop-casted in the channel. This device was further dried under infrared lamp for 30 min and used for the further sensing experiments.Electrical characterization
The two probe device is connected to Keithley Sourcemeter 2612A through copper clips which act as electrodes in the study. The source meter is connected to a computer with the help of GPIB 488A interface.Results and discussion
Structural and morphological analysis
Fig. 1 shows the typical schematic of 2D structure of SnS2 (a) top view and (b) side view respectively. Fig. 2(a) shows the typical X-ray diffraction to investigate the structural properties of SnS2. The sharp and intense peaks confirmed the formation of hexagonal structure of SnS2. The peaks were indexed according to JCPDS data card number 23-0677.The Raman spectroscopy analysis was done to study the signature of vibrational modes to confirm the formation of the SnS2 material. In case of SnS2, the Raman active vibrational mode A1g corresponding to vertical out-of plane vibration between S–S and Eg correspond to non-degenerate in-plane vibration mode.32,35 From Fig. 2(b), it is observed that the Eg mode at ∼224 cm−1 and an intense A1g mode at ∼310 cm−1 for SnS2 nanoflakes. The inset of Fig. 2(b) shows the schematic of the Raman active modes.
Fig. 3(a) and (b) shows the typical low magnification TEM images of as synthesized sample which shows the formation of 2D nanoflake like morphology. The formation of nanoflake morphology occurs due to the hydrolysis and dissociation of the precursors during the hydrothermal reaction. According to Butler et al.50 alkali-assisted hydrolysis of TAA is quicker than acid catalysed hydrolysis. Hence, in our study we used aqueous NaOH solution to assist the hydrolysis of TAA. The proposed mechanism can be presumably explained from the below mentioned equations. Firstly, SnCl4·5H2O dissociates to give Sn4+ ions.
| SnCl4·5H2O → Sn4+ + 4Cl− + 5H2O | (1) |
| CH3CSNH2 + OH− → CH3COO− + HS− | (2) |
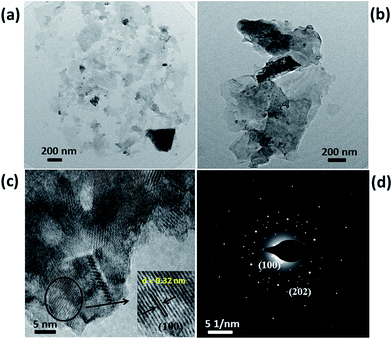 | ||
| Fig. 3 TEM analysis of SnS2 nanoflakes (a and b) low magnification TEM images, (c) high resolution TEM image and (d) SAED pattern. | ||
The HS− will further dissociate as:
| 2HS− → H2S + S2− | (3) |
Further Sn4+ and S2− react to form SnS2.
| Sn4+ + S2− → SnS2 | (4) |
But at lower temperature, the production of S2− is very slow. Hence, subjecting to higher temperature and prolonged time was done for easier and effective nucleation, growth and formation of SnS2 nanoflakes.35,50
Fig. 3(c) shows the high resolution TEM image of SnS2 nanoflake. This was taken to study the lattice fringes and the grain boundaries. A d-spacing of ∼0.32 nm was observed from the HRTEM image as shown in inset of Fig. 3(c). This value can be attributed to (100) plane of SnS2. The SAED pattern of SnS2 nanoflake sample were recorded and shown in Fig. 3(d) which confirms that the synthesized SnS2 sample belongs to the hexagonal crystal structure.
Humidity and alcohol sensor
The humidity sensing analysis was done at relative humidity ranging from 11–97%. Fig. 4(a) shows the schematic of a two-probe SnS2 sensor device used to test the humidity sensing performance. The current–voltage (I–V) measurements were taken from −3 V to +3 V and the resistance values were calculated from the slope of the plot. Fig. 4(b) shows the plot of typical resistance of the sensor device against the relative humidity values. From this plot we observed that the resistance decreased with increasing humidity, establishing that the conductivity increased as the relative humidity increased. These resistance values were also used to calculate the sensitivity of the device. Fig. 4(c) shows the sensitivity vs. relative humidity plot. The % sensitivity has been calculated using the formula:
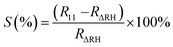 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300% in 97% relative humidity was observed. The repeated cycles of the two-probe sensor device in 11% and 97% relative humidity at a bias voltage +3 V were recorded to study the response and recovery time and cyclic stability of the device. Fig. 4(d) shows the current–time (I–t) plot through which the response and recovery time were noted as ∼85 s and ∼6 s respectively. The fabricated device displayed a quick response upon exposure to the water molecules at room temperature. Note that the response time was much greater than the recovery time. The response of the sensors device is limited by strong adsorption/desorption of analyte molecules at room temperature. The thermodynamic adsorption of analyte molecules may not be much favorable during the response time and during the recovery time analyte molecules easily get desorbs due to low absorption energy, that's why response time was much greater than the recovery time. Further, the response of sensor device may be improved by operating device at slight higher temperature or by light irradiation. The sensitivity can also be modulated using the micro/nano structures of SnS2 nanoflake, smaller the thickness, higher will be the sensitivity due to large surface area. The sensor device was exposed to water molecules at higher relative humidity until a saturation point was achieved. Once the device reached the saturation, the flow of water molecules were turned off by again exposing it to the lower humidity level, so as to attend the original resistance. Though the response time is not the best, the recovery kinetics was much better than many 2D layered materials reported in the literature.11,25,26,48,50–52 This observation might be related to the thickness dependant properties of the SnS2 nanosheets. The comparison of sensing characteristics with other materials has been elaborated in Table 1.
300% in 97% relative humidity was observed. The repeated cycles of the two-probe sensor device in 11% and 97% relative humidity at a bias voltage +3 V were recorded to study the response and recovery time and cyclic stability of the device. Fig. 4(d) shows the current–time (I–t) plot through which the response and recovery time were noted as ∼85 s and ∼6 s respectively. The fabricated device displayed a quick response upon exposure to the water molecules at room temperature. Note that the response time was much greater than the recovery time. The response of the sensors device is limited by strong adsorption/desorption of analyte molecules at room temperature. The thermodynamic adsorption of analyte molecules may not be much favorable during the response time and during the recovery time analyte molecules easily get desorbs due to low absorption energy, that's why response time was much greater than the recovery time. Further, the response of sensor device may be improved by operating device at slight higher temperature or by light irradiation. The sensitivity can also be modulated using the micro/nano structures of SnS2 nanoflake, smaller the thickness, higher will be the sensitivity due to large surface area. The sensor device was exposed to water molecules at higher relative humidity until a saturation point was achieved. Once the device reached the saturation, the flow of water molecules were turned off by again exposing it to the lower humidity level, so as to attend the original resistance. Though the response time is not the best, the recovery kinetics was much better than many 2D layered materials reported in the literature.11,25,26,48,50–52 This observation might be related to the thickness dependant properties of the SnS2 nanosheets. The comparison of sensing characteristics with other materials has been elaborated in Table 1.
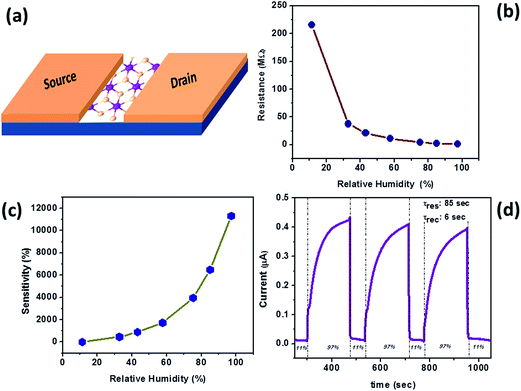 | ||
| Fig. 4 Humidity sensing performance of SnS2 nanoflakes (a) schematic of device, (b) resistance vs. relative humidity plot, (c) sensitivity vs. relative humidity plot and (d) typical I–t cycle plot. | ||
| Materials | Synthesis method | Response time (s) | Recovery time (s) | Reference |
|---|---|---|---|---|
| MoS2 | Mechanical exfoliation | 9 | 17 | 12 |
| WS2 | Hot wire-CVD | 12 | 13 | 16 |
| VS2 | Liquid exfoliation | 30–40 | 12–50 | 52 |
| Black phosphorus | Liquid exfoliation | 255 | 10 | 48 |
| Black phosphorus | Electrochemical exfoliation | 101 | 26 | 25b |
| V2O5 | Hydrothermal method | 240 | 300 | 49 |
| SnS2 | Hydrothermal method | 85 | 6 | This work |
The sensing mechanisms were explained as below. The resistance of the sensor device was recorded at a lower humidity level (11% RH) as the base value resistance. Typically the sensor was then exposed at a different humidity levels from 11% RH to 97% RH with constant flow rate of 10 sccm Ar. When the SnS2 sensor device was exposed to the water molecules, a charge transfer between the water molecules and SnS2 nanosheet takes place which results in the resistance change of the sensor device. The strong interactions between the water molecules and SnS2 nanosheets results into the significant change in conductivity of SnS2 sensor device.51 Water molecule is an electron donating molecule, it possesses n-type characteristics and upon exposure to SnS2 nanosheet sensor device, it injects charges to the surface of sheet and which results into the shifting of Fermi level towards the conduction band of SnS2 leading to the decrease in the resistance of the sensor device.51 Also, the sensing mechanism here is dominant by proton conduction. When the device is exposed to the humid environment, water molecules in its atmosphere get adsorbed on the channel material. At low humidity, less water molecules are adsorbed causing lesser surface interactions with the material and therefore less proton transfer. At higher humidity, due to the adsorption of multiple water layers, the transfer of protons easier and more in number. Hence, the conductivity increases as the proton conduction increases i.e. as relative humidity increases.25,53,54
We also investigated the alcohol sensing behavior of the SnS2 nanoflakes for three analytes methanol, ethanol and isopropyl alcohol (IPA). Fig. 5(a) and (b) shows the response and resistance of the material when the test analyte molecules were introduced in the test chamber. The response was calculated using the following formula,
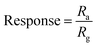 | (6) |
| Alcohols | Response (Ra/Rg) at 150 ppm | Response time (s) | Recovery time (s) |
|---|---|---|---|
| IPA | 85 | 50 | 23 |
| Ethanol | 211 | 69 | 4 |
| Methanol | 1580 | 67 | 5 |
We have thus confirmed the highest selectivity for the methanol, which is probable due to the fact that dissimilar dielectric constants values of these alcohols. For example for the case of methanol the dielectric constant value is ∼32.7, for ethanol it is ∼24.6 and for IPA its ∼18.6.55–57 Thus, it is expected that the response of SnS2 nanosheet sensor device slightly differs upon the adsorption of methanol, ethanol and IPA vapors, thus getting different responses.
Conclusion
In conclusion, we have successfully synthesized 2D SnS2 nanoflakes by one-step hydrothermal synthesis method. The XRD, Raman spectroscopy, TEM, HRTEM and SAED confirmed the hexagonal crystal structure and nanoflake like morphology of SnS2. Further, humidity sensing performance for the 2D SnS2 nanoflakes showed the maximum sensitivity of ∼11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300% in 97 RH%. Our humidity sensor device shows fast response time of ∼85 s and record low recovery time of ∼6 s respectively. We have also studied the alcohol sensing properties of SnS2 nanoflakes for methanol, ethanol and IPA. Compared to ethanol and IPA, methanol showed higher response of ∼1580 at 150 ppm due to high dielectric constant value. The SnS2 based alcohol sensor device shows a response time of ∼67 s and quick recovery time of ∼5 s in case of methanol.
300% in 97 RH%. Our humidity sensor device shows fast response time of ∼85 s and record low recovery time of ∼6 s respectively. We have also studied the alcohol sensing properties of SnS2 nanoflakes for methanol, ethanol and IPA. Compared to ethanol and IPA, methanol showed higher response of ∼1580 at 150 ppm due to high dielectric constant value. The SnS2 based alcohol sensor device shows a response time of ∼67 s and quick recovery time of ∼5 s in case of methanol.
Conflict of interest
The author declares no competing financial interest.Acknowledgements
The research work was supported by Department of Science and Technology (Government of India) under Ramanujan Fellowship (Grant No. SR/S2/RJN-130/2012), NCL-MLP project grant 028626, DST-SERB Fast-track Young scientist project Grant No. SB/FT/CS-116/2013, Broad of Research in Nuclear Sciences (BRNS) Grant No. 34/14/20/2015 (Government of India), partial support by INUP IITB project sponsored by DeitY, MCIT, Government of India.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. S Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef PubMed.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- N. Zhang, Y. Zhang and Y. J. Xu, Nanoscale, 2012, 4, 5792–5813 RSC.
- L. K. Putri, W. J. Ong, W. S. Chang and S. P. Chai, Appl. Surf. Sci., 2015, 358, 2–14 CrossRef CAS.
- F. Bonaccorso, Z. Sun, T. Hasan and A. C. Ferrari, Nat. Photonics, 2010, 4, 611–622 CrossRef CAS.
- V. Yong and J. M. Tour, Small, 2010, 6, 313–318 CrossRef CAS PubMed.
- M. F. El-Kady and R. B. Kaner, Nat. Commun., 2013, 4, 1475 CrossRef PubMed.
- A. Ghosh, D. J. Late, L. S. Panchakarla, A. Govindraj and C. N. R. Rao, J. Exp. Nanosci., 2009, 4, 313–322 CrossRef CAS.
- C. H. Lu, H. H. Yang, C. L. Zhu, X. Chen and G. N. Chen, Angew. Chem., 2009, 121, 4879–4881 CrossRef.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotech., 2011, 6, 147–150 CrossRef CAS PubMed.
- D. J. Late, Y. K. Huang, B. Liu, J. Acharya, S. N. Shirodkar, J. Luo, A. Yan, D. Charles, U. V. Waghmare, V. P. Dravid and C. N. R. Rao, ACS Nano, 2013, 7, 4879–4891 CrossRef CAS PubMed.
- D. J. Late, B. Liu, H. S. S. R. Matte, V. P. Dravid and C. N. R. Rao, ACS Nano, 2012, 6, 5635–5641 CrossRef CAS PubMed.
- R. S. Sundaram, M. Engel, A. Lombardo, R. Krupke, A. C. Ferrari, P. Avouris and M. Steiner, Nano Lett., 2013, 13, 1416–1421 CrossRef CAS PubMed.
- D. Ovchinnikov, A. Allain, Y.-S. Huang, D. Dumcenco and A. Kis, ACS Nano, 2014, 8, 8174–8181 CrossRef CAS PubMed.
- A. S. Pawbake, R. Waykar, D. J. Late and S. R. Jadkar, ACS Appl. Mater. Interfaces, 2016, 8, 3359–3365 CAS.
- Y. Chang, W. Zhang, Y. Zhu, Y. Han, J. Pu, J. Chang, W. Hsu, J. Huang, C. Hsu, M. Chiu, T. Takenobu, H. Li, C. Wu, W. Chang, A. T. S. Wee and L. Li, ACS Nano, 2014, 8, 8582–8590 CrossRef CAS PubMed.
- S. Larentis, B. Fallahazad and E. Tutuc, Appl. Phys. Lett., 2012, 101, 223104 CrossRef.
- S. Das and J. Appenzeller, Appl. Phys. Lett., 2013, 103, 103501 CrossRef.
- A. Allain and A. Kis, ACS Nano, 2014, 8, 7180–7185 CrossRef CAS PubMed.
- J. Huang, J. Pu, C. Hsu, M. Chiu, Z. Juang, Y. Chang, W. Chang, Y. Iwasa, T. Takenobu and L. Li, ACS Nano, 2014, 8, 923–930 CrossRef CAS PubMed.
- (a) X. Zhou, L. Gan, W. Tian, Q. Zhang, S. Jin, H. Li, Y. Bando, D. Golberg and T. Zhai, Adv. Mater., 2015, 27, 8035–8041 CrossRef CAS PubMed; (b) A. S. Pawbake, A. Date, S. R. Jadkar and D. J. Late, Chemistry Select, 2016, 1, 5380–5387 CAS.
- (a) L. Zhou, K. Xu, A. Zubair, A. D. Liao, W. Fang, F. Ouyang, Y. Lee, K. Ueno, R. Saito, T. Palacios, J. Kong and M. S. Dresselhaus, J. Am. Chem. Soc., 2015, 137, 11892–11895 CrossRef CAS PubMed; (b) D. J. Late, Applied Materials Today, 2016, 5, 98–102 CrossRef.
- M. N. Ali, J. Xiong, S. Flynn, J. Tao, Q. D. Gibson, L. M. Schoop, T. Liang, N. Haldolaarachchige, M. Hirschberger, N. P. Ong and R. J. Cava, Nature, 2014, 514, 205–208 CAS.
- (a) L. Li, Y. Yu, G. J. Ye, Q. Ge, X. Ou, H. Wu, D. Feng, X. H. Chen and Y. Zhang, Nat. Nanotechnol., 2014, 9, 372–377 CrossRef CAS PubMed; (b) M. B. Erande, M. S. Pawar and D. J. Late, ACS Appl. Mater. Interfaces, 2016, 8, 11548–11556 CrossRef CAS PubMed.
- (a) J. O. Island, M. Buscema, M. Barawi, J. M. Clamagirand, J. R. Ares, C. Sánchez, I. J. Ferrer, G. A. Steele, H. S. J. van der Zant and A. Castellanos-Gomez, Adv. Opt. Mater., 2014, 2, 641–645 CrossRef CAS; (b) A. S. Pawbake, J. O. Island, E. Flores, J. R. Ares, C. Sanchez, I. J. Ferrer, S. R. Jadkar, H. S. van der Zant, A. Castellanos-Gomez and D. J. Late, ACS Appl. Mater. Interfaces, 2015, 7, 24185–24190 CrossRef CAS PubMed.
- Y. C. Zhang, Z. N. Du, K. W. Li and M. Zhang, Sep. Purif. Technol., 2011, 81, 101–107 CrossRef CAS.
- L. A. Burton and A. Walsh, J. Phys. Chem. C, 2012, 116, 24262–24267 CAS.
- T. J. Kim, C. Kim, D. Son, M. Choi and B. Park, J. Power Sources, 2007, 167, 529–535 CrossRef CAS.
- L. A. Burton and A. Walsh, J. Phys. Chem. C, 2012, 116, 24262–24267 CAS.
- M. He, L. X. Yuan and Y. H. Huang, RSC Adv., 2013, 3, 3374–3383 RSC.
- J. Z. Ou, W. Ge, B. Carey, T. Daeneke, A. Rotbart, W. Shan, Y. Wang, Z. Fu, A. F. Chrimes, W. Wlodarski, S. P. Russo, Y. X. Li and K. Kalantar-Zadeh, ACS Nano, 2015, 9, 10313–10323 CrossRef CAS PubMed.
- K. T. Lee, Y. C. Liang, H. H. Lin, C. H. Li and S. Y. Lu, Electrochim. Acta, 2016, 219, 241–250 CrossRef CAS.
- X. Cui, W. Xu, Z. Xie and Y. Wang, J. Mater. Chem. A, 2016, 4, 1908–1914 CAS.
- C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Appl. Phys. Lett., 2014, 105, 043109 CrossRef.
- Z. Yang, Y. Ren, Y. Zhang, J. Li, H. Li, X. H. Hu and Q. Xu, Biosens. Bioelectron., 2011, 26, 4337–4341 CrossRef CAS PubMed.
- J. Li, Z. Yang, Y. Tang, Y. Zhang and X. Hu, Biosens. Bioelectron., 2013, 41, 698–703 CrossRef CAS PubMed.
- J. Li, Z. Yang, Y. Zhang, S. Yu, Q. Xu, Q. Qu and X. Hu, Microchim. Acta, 2012, 179, 265–272 CrossRef CAS.
- A. Yella, E. Mugnaioli, H. A. Therese, M. Panthöfer, U. Kolb and W. Tremel, Chem. Mater., 2009, 21, 2474–2481 CrossRef CAS.
- Y. T. Lin, J. B. Shi, Y. C. Chen, C. J. Chen and P. F. Wu, Nanoscale Res. Lett., 2009, 4, 694–698 CrossRef CAS PubMed.
- D. Gao, Q. Xue, X. Mao, M. Xue, S. Shi and D. Xue, CrystEngComm, 2014, 16, 7876–7880 RSC.
- L. Zhuo, Y. Wu, L. Wang, Y. Yu, X. Zhang and F. Zhao, RSC Adv., 2012, 2, 5084–5087 RSC.
- Y. Poya, A. Behranginia, T. Foroozan, M. Asadi, K. Kim, F. K. Araghi and A. Salehi-Khojin, ACS Nano, 2015, 9, 9898–9905 CrossRef PubMed.
- D. Chen, X. Hou, H. Wen, Y. Wang, H. Wang, X. Li, R. Zhang, H. Lu, H. Xu, S. Guan, J. Sun and L. Gao, Nanotechnology, 2010, 21, 035501 CrossRef PubMed.
- C. C. Mayorga-Martinez, Z. Sofer and M. Pumera, Angew. Chem., Int. Ed., 2015, 127, 14525–14528 CrossRef.
- S. L. Zhang, H. Yue, X. Liang and W. C. Yang, J. Nanosci. Nanotechnol., 2015, 15, 8004–8009 CrossRef CAS PubMed.
- C. C. Mayorga-Martinez, A. Ambrosi, A. Y. S. Eng, Z. Sofer and M. Pumera, Adv. Funct. Mater., 2015, 25, 5611–5616 CrossRef CAS.
- D. J. Late, Microporous Mesoporous Mater., 2016, 225, 494–503 CrossRef CAS.
- M. S. Pawar, P. K. Bankar, M. A. More and D. J. Late, RSC Adv., 2015, 5, 88796–88804 RSC.
- E. A. Butler, D. G. Peters and E. H. Swift, Anal. Chem., 1958, 30, 1379–1383 CrossRef CAS.
- H. Hassan, J. Mun, B. S. Kang, J. Y. Song, T. Kim and S. Kang, RSC Adv., 2016, 6, 75839–75843 RSC.
- J. Feng, L. Peng, C. Wu, X. Sun, S. Hu, C. Lin, J. Dai, J. Yang and Y. Xie, Adv. Mater., 2012, 24, 1969–1974 CrossRef CAS PubMed.
- Z. Chen and C. Lu, Sens. Lett., 2005, 3, 274–295 CrossRef CAS.
- B. Chitara, D. J. Late, S. B. Krupanidhi and C. N. R. Rao, Solid State Commun., 2010, 150, 2053–2057 CrossRef CAS.
- Common Organic Solvents: Table of Properties: https://www.organicdivision.org/orig/organic_solvents.html, accessed: Oct. 2016.
- P. Yasaei, A. Behranginia, T. Foroozan, M. Asadi, K. Kim, F. Khalili-Araghi and A. Salehi-Khojin, ACS Nano, 2015, 9, 9898–9905 CrossRef CAS PubMed.
- C. C. Mayorga-Martinez, Z. Sofer and M. Pumera, Angew. Chem., Int. Ed., 2015, 54, 14317–14320 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21252b |
| This journal is © The Royal Society of Chemistry 2016 |



