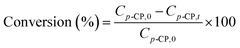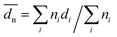The reaction mechanism for highly effective hydrodechlorination of p-chlorophenol over a Pd/CNTs catalyst†
Lijuan Lanab,
Fanglin Du*a and
Chuanhai Xia*c
aCollege of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: fldu@qust.edu.cn; Tel: +86 532 84022870
bSchool of Environment and Materials Engineering, Yantai University, Yantai 264005, China
cSchool of Resources and Environmental Engineering, Ludong University, Yantai 264025, China. E-mail: chxia_ldu@hotmail.com; Tel: +86 535 6016605
First published on 31st October 2016
Abstract
Carbon nanotubes (CNTs) supported Pd catalysts (1 wt% and 5 wt%) were prepared by a conventional impregnation method and tested in liquid-phase hydrodechlorination (HDC) of para-chlorophenol (p-CP) under mild conditions (313 K and atmospheric pressure) with H2. CNTs as a catalyst support offered better performance than activated carbon (AC). The conversion of p-CP reached 72% and 82% over 5 wt% Pd/AC and 5 wt% Pd/CNTs, respectively, within 30 min. When the content of Pd was reduced to 1 wt%, the conversion of p-CP over Pd/AC and Pd/CNTs was 55% and 83%, respectively. The mechanism of this phenomenon was studied through catalyst characterization (XRD, TEM, and BET). The abovementioned results indicated that the different activities of the catalysts over different supports mainly resulted from the porous structure of the supports and nanoparticle diameters of the active metal.
1. Introduction
Chlorophenols (CPs) have been used extensively for the synthesis of pesticides, medicine, dye, plastic, and ethanol alterant. However, they have been listed as priority pollutants by the US Environmental Protection Agency (EPA) and European regulatory authorities due to their high toxicity, persistence and low biodegradability.1 Therefore, it is an urgent task to develop effective treatment methods for the degradation of CPs. Recently, CPs are mainly being destroyed by bioremediation,2,3 photochemical degradation,4,5 irradiation,6,7 oxidative degradation,8,9 metal-mediated reductive dechlorination,10,11 and catalytic hydrodechlorination (HDC).12–14 Unfortunately, most of these methods require rigorous reaction conditions with special equipment, and/or many of them are impractical to scale-up. Among the above mentioned methods, catalytic HDC is an environmentally friendly method, which can convert organochlorinated pollutants into recyclable raw materials with low energy consumption and can also prevent the generation of toxic by-products under relatively mild conditions.In general, catalytic HDC is carried out using supported metal catalysts such as Pt,15,16 Ni,17,18 Rh,19,20 Ru,18,21,22 and Pd.19,23,24 Among these supported metal catalysts, Pd catalyst is widely used owing to its high catalytic activity, resistance to the attack of acids and bases, and relative stability under severe conditions.25,26 In addition to active metals, supports also play an important role in the HDC reaction. To date, various supports have been developed, such as active carbon (AC),15,19,21,27 Al2O3,30–33 pillared clays,26,34 SiO2,30–33 zeolite,35 and ZrO2,36 for catalytic HDC. Among these, AC is regarded as a conventional catalyst support used in industry due to its huge specific surface area and high stability, which is beneficial to the HDC reaction and the recovery of metal elements. Recently, other carbon materials, such as carbon nanofibers28–30 and graphites,28,29 have received increasing attention in the fields of environmental and industrial heterogeneous catalysis. Since their discovery by Iijima in 1991, CNTs have led to a growing interest in nanocomposites, synthesis, and pharmaceutics owing to their unique chemical structures and one-dimensional macromolecules that possess extraordinary electrical, thermal and chemical stability.37 The unique structural features and electronic properties have attracted a lot of research work in the use of CNTs as adsorbing materials,38,39 particularly as hydrogen storage materials40–42 and catalyst supports.43–45 In numerous heterogeneous catalytic reactions,46,47 CNTs as catalyst supports exhibited much better activity and selectivity compared with the conventional supports. Singh et al.40 have reported that transition metal atoms, such as Pd, Pt, and Ni could be stabilized on sp2-hybridized carbon atoms, inducing multiple bonding of molecular hydrogen with adsorption energies intermediate between physisorption and chemisorption.
Many supported catalysts, which used CNTs as the support, exhibited good catalytic activity in the hydrogenation reaction; however, CNTs as the support have received little attention in the liquid-phase HDC. In our previous work,48–51 Pd/AC catalysts exhibited a high catalytic activity in liquid-phase HDC. Herein, AC and CNTs were chosen to study the role of the supports. Pd/CNTs and Pd/AC catalysts were prepared and their catalytic performance was compared in the liquid-phase HDC of p-CP. To investigate the influence of catalyst supports, samples of the catalysts were analyzed by ICP, XRD, TEM, SEM, and BET methods.
2. Experimental
2.1 Chemicals
AC (20–50 mesh) used in the study was purchased from Aladdin Industrial Corporation, Shanghai, China. The multi-carbon nanotubes (layers: 8–15; length: 0.5–5 μm) were obtained from Shandong Dazhan Nano Materials Co., Ltd. p-CP was purchased from Aladdin Industrial Corporation, Shanghai, China. The other regents, such as nitric acid, base and solvents, were of analytical grade and were supplied by Sinopharm Chemical Regent Co., Ltd., Shanghai, China. Deionized water was used in the reaction. The purity of hydrogen gas used in the experiments was more than 99.99%.2.2 Catalyst preparation
The AC (which was grinded to a 200 mesh) and CNTs were contacted (agitation at 300 rpm) with nitric acid in an oil bath for 10 h at 353 K. Concentrated hydrochloric acid was added to increase the solubility of PdCl2 in an aqueous solution. Then, the mixture was diluted with deionized water, followed by filtration, thorough washing with an excess of water (until pH approached 7) and oven drying at 373 K for 12 h to obtain the acidified AC and CNTs.5 g of acidified AC and CNTs were added to a 100 mL vessel with certain amount of water, sonicated in a bath for 30 min, a PdCl2 solution (with the loading of Pd) was added dropwise at 303 K with constant agitation (100 rpm), and placed at room temperature for 2 h. Then, formaldehyde and NaOH solutions were added dropwise to the mixture with constant agitation (100 rpm) as the Pd-based catalyst was prepared by reduction with formaldehyde as a reducing agent under alkaline conditions. After standing over 6 h, the mixture was filtered, washed with distilled water repeatedly to remove chloridion in the final product, and then oven-dried at 373 K for 24 h, to obtain the Pd/AC and Pd/CNTs catalysts.
The Pd loading was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Vista-PRO, Varian Inc.).
2.3 Catalytic procedure
The HDC of p-CP was carried out in a 100 mL three-necked flask with magnetic stirring at 1200 rpm. The reaction vessel was placed in a temperature-controlled heating water bath with a precision of ±1 K. p-CP was treated with hydrogen gas over some amount of supported Pd catalyst in water solutions under mild conditions (313 K and atmospheric pressure). H2 flow (10 mL min−1) and stirring speed (1200 rpm) served to minimize the external H2 transport limitations. The stability of the catalysts was tested under the same conditions. In this study, although the concentration of p-chlorophenol in the reaction solution was high (5.0 g L−1, 400 mg in 80 mL solution), the amount of Pd catalyst in the reaction solution was only 30 mg. Thus, the effect of adsorption in the absence of H2 would be negligible.The composition of the reaction system was determined by HPLC (Agilent 1200) equipped with a chromatographic column (XDB-C18: 4.6 × 150 mm, 5 μm). The conversion of p-CP was applied to account for the catalytic activity of the catalysts in the HDC reaction, which is defined as follows:
2.4 Catalyst characterization
Powder X-ray diffraction (XRD) pattern of the catalyst was obtained using an XRD-7000S/L of Shimadzu with a Cu Kα radiation at 40 kV and 30 mA. The samples were scanned at a rate of 0.1° s−1 over the 10° ≤ 2θ ≤ 80° range with a scan time of 5 s per step.The morphology of the catalyst was characterized using transmission electron microscopy (TEM, a JEOL Mode HEM-2011 EM, Japan) at an accelerating voltage of 120 kV. The crushed catalysts were prepared first by dispersing in ethanol, and then by depositing two drops of the dispersed samples on a copper mesh grid. At least 300 individual Pd particles were counted for each catalyst. The mean Pd particle sizes as a number averaged diameter are quoted in this paper:
Scanning electron microscopy (SEM) was conducted using a Hitachi S4800 field emission SEM, operated at an accelerating voltage of 20 kV; specific BET surface area and BJH pore volume analyses were performed using the commercial Micrometrics ST2000B unit; N2 at 77 K served as sorbate. Before measurement, the samples were outgassed at 433 K for 16 h.
3. Results and discussion
3.1 Catalyst characterization: XRD and ICP
XRD patterns scanned over angles (10–80°) are given in Fig. 1. Characteristic peaks of carbon for AC and CNTs observed at 2θ values of 26°, 43°, and 53° were, respectively, identified to diffractions of (0 0 2), (1 0 0), and (0 0 4) planes.52 The peaks at 2θ values of 40°, 46°, and 68° in 5 wt% Pd/AC and 5 wt% Pd/CNTs can be assigned to (1 1 1), (2 0 0), and (2 2 0) crystalline Pd planes, respectively, which were consistent with the PDF standard card of Pd (JCPDS 05-0681).53 According to the XRD patterns of 5% Pd/CNTs and 5% Pd/AC, it would be reasonable to presume that the catalyst active component had been successfully loaded on AC and CNTs. | ||
| Fig. 1 (a) XRD patterns for (a) CNTs, (b) 1% Pd/CNTs and (c) 5% Pd/CNTs, (b) XRD patterns for (a) AC, (b) 1% Pd/AC and (c) 5% Pd/AC. | ||
For 1 wt% Pd/CNTs catalysts, the corresponding diffraction peak of Pd was only found at 2θ values of 40° (Fig. 1a), which was weakened in contrast to the XRD pattern of 5 wt% Pd/CNTs. Similarly, the XRD pattern of 1 wt% Pd/AC exhibited a weak peak at 2θ values of 40° (Fig. 1b). The weakened Pd peak of XRD patterns is possibly due to the low Pd loading amounts in 1 wt% Pd/CNTs and Pd/AC.
To evaluate the actual loading amounts of Pd on CNTs and AC, ICP-MS analysis of Pd/AC and Pd/CNTs was carried out, and the actual loading amounts of Pd on AC and CNTs were 5.01%, 0.98%, 4.97%, and 1.03%, respectively. This suggested that the actual loading amounts of Pd were almost the same as the theoretical values for the loading amounts of Pd on AC and CNTs. The results demonstrated that Pd active component had been successfully loaded on AC and CNTs.
3.2 Catalytic activity of Pd/AC and Pd/CNTs
To evaluate the catalytic activity of 5 wt% Pd/AC and 5 wt% Pd/CNTs, catalytic HDC of p-CP was performed at 313 K under atmospheric pressure. The activity profiles presented the conversion of p-CP within the reaction time over both 5 wt% Pd/CNTs and 5 wt% Pd/AC catalysts (Fig. 2). In the first 30 min, the conversion of p-CP reached 72% and 82% over 5 wt% Pd/AC and 5 wt% Pd/CNTs catalysts, respectively, which meant that the activity of the two catalysts was very high. However, as the HDC reaction proceeded, the conversion increased slowly, and the conversion of p-CP over 5 wt% Pd/AC and 5 wt% Pd/CNTs catalysts at 60 min was 96% and 95%, respectively.As shown in Fig. 2, the conversion rate of p-CP over both 5 wt% Pd/AC and 5 wt% Pd/CNTs was high with no obvious difference. The high activity of the two catalysts might be due to the high loading of Pd. Thus, 1 wt% Pd/CNTs and 1 wt% Pd/AC catalysts were chosen to investigate the activities of the catalysts (Fig. 3). As illustrated in Fig. 3, the conversion of p-CP was 83% over 1 wt% Pd/CNTs in the first 30 minutes, whereas the conversion of p-CP was only 55% over 1 wt% Pd/AC within the same reaction time. Although, the conversion of p-CP over 1 wt% Pd/CNTs reached 100% within 50 min, the conversion of p-CP over 1 wt% Pd/AC was only 83%. When the content of Pd was reduced from 5 wt% to 1 wt%, the activity difference between the Pd/CNTs and Pd/AC catalysts became obvious (Fig. 3). Compared to the Pd catalysts on other supports and those reported, it was found that the average HDC rates over Pd/CNTs catalysts in the study were much higher than those of the Pd catalysts on other supports and those reported in the literature (Table S1 in ESI†). This indicated that Pd/CNTs exhibited much higher catalytic activity in the HDC of p-CP.
Subsequently, catalytic HDC of p-CP was repeated 3 times with the catalyst recovered after each reaction to evaluate the stability of 5 wt% and 1 wt% Pd/CNTs (Fig. 4). As shown in Fig. 4, the conversion of p-CP within 60 min over 5 wt% Pd/CNTs was 95% for the first time, and then dropped to 90% in the 3rd run. However, for 1 wt% Pd/CNTs, the conversion of p-CP within 50 min was 100% for the first time, and then dropped to 94% in the 3rd run. It can be observed that the activity of Pd/CNTs catalyst gradually decreased with the increase in the reused time but the damping was not obvious. These results suggested that Pd/CNTs possessed a high activity and stability and could be reused for at least 3 times for the HDC of p-CP under mild conditions.
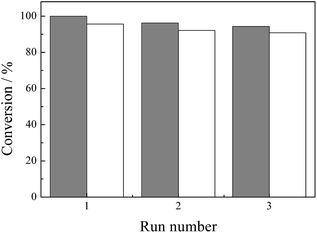 | ||
| Fig. 4 Repeated HDC of p-CP over 1% Pd/CNTs for 50 min (filled square) (reaction conditions are the same as mentioned in Fig. 2) and 5% Pd/CNTs for 60 min (open square) (reaction conditions are the same as mentioned in Fig. 1). | ||
The catalysts exhibited different catalytic activities in the HDC of p-CP when AC and CNTs were used as the catalyst supports. Why was the activity higher with CNTs as the catalyst support? Singh et al.40 have reported that Pd could be stabilized on sp2-hybridized carbon atoms. What was the other effect of CNTs as a support on the catalytic performance? To obtain more accurate evidence for the roles of AC and CNTs, TEM, SEM, BET-pore analysis were performed in the following research.
3.3 Catalyst characterization: TEM and SEM
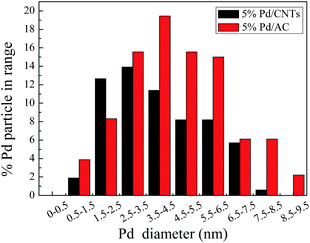
The characteristics of the supports is one of the important factors that affect the dispersion and nanoparticle sizes of Pd as the catalyst active component, which plays an important role in the HDC reaction.22,54 Thus, catalyst characterizations (TEM, SEM, and BET) were introduced to analyze the samples of Pd/AC and Pd/CNTs catalysts.First, fresh Pd/AC and Pd/CNTs catalysts were analyzed by TEM. The representative TEM images are given in Fig. 5, and the TEM images for the particle size distribution of are provided in Fig. 6 and 7. As illustrated in Fig. 5B and D, metallic Pd nanoparticles were randomly distributed on the external wall of CNTs. It can be seen from Fig. 6 and 7 that 5 wt% and 1 wt% Pd/AC catalysts yielded metal particles of around 1–12 nm, whereas 5 wt% and 1 wt% Pd/CNTs catalysts yielded metal particle of around 1–7 nm (most of them in 3–6 nm range). Moreover, Pd/CNTs catalysts were characterized to have a small average diameter (2.60 nm for 1 wt% Pd/CNTs versus 4.30 nm for 5 wt% Pd/CNTs), and a narrow particle size distribution (more than 70% of the total particles number within 3 nm for 1 wt% catalyst, and more than 50% of the total particles number within 5 nm for 5 wt% catalysts). However, Pd/AC catalysts presented a large average diameter (6.85 nm for 1 wt% Pd/AC versus 8.31 nm for 5 wt% Pd/AC) and an extensive particle size distribution (Table 1). Moreover, it can be observed from Table 1 that the dispersion of Pd on CNTs for 5 wt% Pd/CNTs and 1 wt% Pd/CNTs catalysts are 48% and 79%, respectively, whereas the dispersion of Pd on AC for 5 wt% Pd/AC and 1 wt% Pd/AC catalysts are 25% and 30%, respectively. This suggested that the dispersion of Pd on CNTs is much higher than that of Pd on AC.
The abovementioned results suggested that the Pd nanoparticles were not well distributed on the AC surface due to agglomeration; however, they were highly dispersed covering large area of the CNTs with minimal aggregation. In general, smaller particle size leads to a large specific surface area, stronger adsorption function and more active sites.22,29 In the HDC of p-CP, Pd/CNTs possessed a small average Pd diameter and a narrow particle size distribution, which enabled the catalyst to retain high activity.
Subsequently, SEM analyses of AC and CNTs were performed to illustrate the role of AC and CNTs in the process of Pd loading (Fig. 8 and 9). As shown in Fig. 8a, AC was in the form of granular stack with the diameter of the granules ranging between 50 and 300 μm. Fig. 8B–D showed that there were numerous holes (the diameters between 0.8 and 2 μm) on the surface, which could explain why AC possessed a large surface area.
 | ||
| Fig. 8 SEM images of the raw material of activated carbon (A) 10 μm, (B) 2 μm, (C) 1 μm, and (D) 200 nm. | ||
 | ||
| Fig. 9 SEM images of the raw material of carbon nanotubes (A) 10 μm, (B) 2 μm, (C) 1 μm, and (D) 200 nm. | ||
The SEM images provided in Fig. 9 showed that the CNTs had totally different surface structures compared to AC. CNTs were composed of fibrous structures with a closed top and hollow inner cavity. The pipe walls of CNTs were made up of multilayer graphene curled up seamless with the diameter between 10 and 30 nm, and its length could be up to the micrometer scale. CNTs catalysts prepared from the conventional impregnation method possessed a certain amount of functional sites on the outer surface, which made it easy for Pd nanoparticles to attach on them (Fig. 9). However, the growth of the Pd nanoparticles was limited on the curved surface, which provided a small actual contact area such that the smaller Pd nanoparticles were obtained on the CNTs catalysts rather than the AC catalysts. Moreover, the Pd nanoparticles could enter into the internal micropores of AC and attach to them. AC exhibited a blocky structure, and it could provide enough area for the Pd nanoparticles to grow into larger particles. That is, the position of the reaction active sites and the loading areas of CNTs and AC were quite different. Mass transfer rate is one of the important factors that affect the HDC rate of p-CP. In the HDC of p-CP, the substrate and H2 could easily be adsorbed onto the outer surface of CNTs because of the little mass transfer resistance. However, there was much transfer resistance for the substrate and H2 to reach into the internal micropores of AC catalysts.
3.4 Catalyst characterization: BET-pore analysis
In addition, BET-pore analysis was carried out to obtain more comprehensive information about the structure of AC and CNTs catalysts. The total pore volume and the average pore radii of 5 wt% Pd/AC and 5 wt% Pd/CNTs are shown in Table 2.The low temperature nitrogen adsorption/desorption isotherms of AC catalysts (displayed in Fig. 10a) were consistent with the type IV (IUPAC classification) adsorption/desorption isotherms. It was convex upward and increased rapidly at low P/P0 area (Dubinin56 called this phenomenon as ‘micropore filling’). This illustrated that AC was indicative of a micropore structure with an estimated 25% of the total pores with radii <2 nm,41 which coincided with the results shown in Fig. 10b. At higher P/P0 (P/P0 > 0.4), the adsorbing capacity tended to attain a constant value with hysteresis loops, which suggested that a small number of mesopores existed in the AC.29
 | ||
| Fig. 10 (a) N2 adsorption/desorption isotherms (at 77 K) for (I) 5% Pd/AC and (II) 5% Pd/CNT. (b) Pore size distribution for (I) 5% Pd/AC and (II) 5% Pd/CNT. | ||
Similarly, CNTs were also consistent with the type IV multilayer adsorption/desorption isotherms. However, CNTs were indicative of a mesoporous structure. At low P/P0 area, the adsorption quantity was little due to the lack of micropores; however, the adsorption/desorption isotherms increased rapidly at higher P/P0 area due to capillary condensation, which generated hysteresis loops. The type of hysteresis loop of CNTs belonged to type A,57 which was characterized by a cylindrical hole and uniform pore size. The result was consistent with the structural characteristics of CNTs, which intertwined together to form mesopores. The pore size distribution in the regions of meso- (from 2 to 50 nm) and macro- (>50 nm) pores (from a BJH analysis) are given in Fig. 10b.
Pd/AC exhibited a high BET surface area (1357 m2 g−1, Table 2), whereas the surface area of Pd/CNTs was only 217 m2 g−1. Although Pd/AC exhibited a higher BET surface area, Pd/CNTs showed a greater adsorption capacity than Pd/AC. Fig. 10a showed that at P/P0 = 1, VN2 adsorbed on AC and CNTs was 600 cm3 g−1 and 1200 cm3 g−1, respectively. At present, the application of CNTs as a hydrogen storage material is a significant research topic. Hydrogen gas could be well adsorbed and stored on CNTs.40–42 When CNTs were used as the catalyst support, it was beneficial for the HDC reaction due to the high concentration of hydrogen gas on it. Moreover, CNTs mainly comprised mesopores and the average pore diameter was about 20 nm. The absence of micropores in the CNTs led to a higher catalytic activity due to the less mass transfer limitation as compared to the AC, which had a microporous structure. The average pore diameter of AC was about 3 nm; however, the presence of a large number of micropores inside AC highly hindered the p-CP and phenol diffusion, which resulted in a lower activity despite the high surface area.
4. Conclusions
In summary, the Pd/AC and Pd/CNTs catalysts were synthesized using the impregnation method. Pd/CNTs catalysts exhibited better catalytic activity than the Pd/AC catalysts in the HDC of p-CP under mild conditions. In this study, the high catalytic activity of Pd observed over Pd/CNTs catalysts might be explained by two major factors: (i) CNTs represented an excellent support with the absence of diffusion and material mass-transfer limitation associated with the mesopores. (ii) Pd/CNTs catalysts possessed a small average Pd diameter and a narrow particle size distribution. Pd/CNTs catalysts could be recovered and reused for 3 times without much loss of catalytic activity. This study has provided a useful catalyst for the HDC reaction, and a further detailed research on the structure–function–activity relationship of Pd/CNTs catalysts is in progress.Acknowledgements
This study was funded and conducted by the Project of the Cultivation Plan of Superior Discipline Talent Teams of Universities in Shandong Province: the Coastal Resources and Environment team for Blue-Yellow Area, the National Science Foundation of China (21377162 and 51272115) and the Natural Science Foundation of Shandong Province (ZR2012EMM001).Notes and references
- B. B. Huang, C. Lei, C. H. Wei and G. M. Zeng, Environ. Int., 2014, 71, 118–138 CrossRef CAS PubMed.
- A. Uysal and A. Turkman, J. Hazard. Mater., 2007, 148, 151–157 CrossRef CAS PubMed.
- F. Y. Kong, A. J. Wang, H. Y. Ren, L. P. Huang, M. Y. Xu and H. C. Tao, Bioresour. Technol., 2014, 158, 32–38 CrossRef CAS PubMed.
- P. Krystynik, P. Kluson, S. Hejda, D. Buzek, P. Masin and D. N. Tito, Appl. Catal., B, 2014, 160–161, 506–513 CrossRef CAS.
- E. D. Mato, F. C. Tamarit, S. Bogialli, D. G. Fresnadillo and M. D. Marazuela, Appl. Catal., B, 2014, 160–161, 445–455 CrossRef.
- S. Yamazaki, Y. Fujiwara, S. Yabuno, K. Adachi and K. Honda, Appl. Catal., B, 2012, 121–122, 148–153 CrossRef CAS.
- H. Q. Sun, Y. Bai, H. J. Liu, W. Q. Jin and N. P. Xu, J. Photochem. Photobiol., A, 2009, 201, 15–22 CrossRef CAS.
- L. Z. Zhang, H. H. Zeng, Y. M. Zeng, Z. H. Zhang and X. F. Zhao, J. Mol. Catal. A: Chem., 2014, 392, 202–207 CrossRef CAS.
- X. L. Hao, M. H. Zhou, Q. Xin and L. C. Lei, Chemosphere, 2007, 66, 2185–2192 CrossRef CAS PubMed.
- R. Cheng, J. L. Wang and W. X. Zhang, J. Hazard. Mater., 2007, 144, 334–339 CrossRef CAS PubMed.
- L. J. Xu and J. L. Wang, Appl. Catal., B, 2013, 142–143, 396–405 CrossRef CAS.
- C. H. Xia, Y. Liu, J. Xu, J. B. Yu, W. Qin and X. M. Liang, Catal. Commun., 2009, 10, 456–458 CrossRef CAS.
- Z. P. Dong, C. X. Dong, Y. S. Liu, X. D. Le, Z. C. Jin and J. T. Ma, Chem. Eng. J., 2015, 270, 215–222 CrossRef CAS.
- X. L. Cui, W. Zuo, M. Tian, Z. P. Dong and J. T. Ma, J. Mol. Catal. A: Chem., 2016, 423, 386–392 CrossRef CAS.
- M. A. Álvarez-Montero, L. M. Gómez-Sainero, A. Mayoral, I. Diaz, R. T. Baker and J. J. Rodriguez, J. Catal., 2011, 279, 389–396 CrossRef.
- Y. X. Han, J. Zhou, W. J. Wang, H. Q. Wan, Z. Y. Xu, S. R. Zheng and D. Q. Zhu, Appl. Catal., B, 2012, 125, 172–179 CrossRef CAS.
- X. X. Ma, S. W. Zhou, C. Y. Yang, S. J. Liu, X. L. Bi and C. H. Xia, Catal. Commun., 2010, 12, 282–285 CrossRef CAS.
- Z. F. Zhao, Z. J. Wu, L. X. Zhou, M. H. Zhang, W. Li and K. Y. Tao, Catal. Commun., 2008, 9, 2191–2194 CrossRef CAS.
- E. Diaz, A. F. Mohedano, J. A. Casas, L. Calvo, M. A. Gilarranz and J. J. Rodriguez, Appl. Catal., B, 2011, 106, 469–475 CrossRef CAS.
- J. A. Baeza, L. Calvo, M. A. Gilarranz and J. J. Rodriguez, Chem. Eng. J., 2014, 240, 271–280 CrossRef CAS.
- T. Yoneda, T. Takido and K. Konuma, Appl. Catal., B, 2008, 84, 667–677 CrossRef CAS.
- C. S. Srikanth, V. P. Kumar, B. Viswanadham and K. V. R. Chary, Catal. Commun., 2011, 13, 169–172 CrossRef.
- J. B. Zhao, W. J. Li and D. R. Fang, RSC Adv., 2015, 53, 42861–42868 RSC.
- J. Zhou, H. Chen, Q. Y. Chen and Z. L. Huang, Appl. Surf. Sci., 2016, 387, 588–594 CrossRef CAS.
- M. Martin-Martinez, L. M. Gómez-Sainero, M. A. Alvarez-Montero, J. Bedia and J. J. Rodriguez, Appl. Catal., B, 2013, 132–133, 256–265 CrossRef CAS.
- C. B. Molina, A. H. Pizarro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2014, 148–149, 330–338 CrossRef CAS.
- M. Munoz, M. Kaspereit and B. J. M. Etzold, Chem. Eng. J., 2016, 285, 228–235 CrossRef CAS.
- C. Amorim, X. D. Wang and M. A. Keane, Chin. J. Catal., 2011, 32, 746–755 CrossRef CAS.
- D. R. Fang, W. J. Li, J. B. Zhao, S. Liu, X. X. Ma, J. G. Xu and C. H. Xia, RSC Adv., 2014, 103, 59204–59210 RSC.
- C. B. Molina, A. H. Pizarro, M. A. Gilarranz, J. A. Casas and J. J. Rodriguez, Chem. Eng. J., 2010, 160, 578–585 CrossRef CAS.
- Z. J. Wu, C. X. Sun, Y. Chai and M. H. Zhang, RSC Adv., 2011, 7, 1179–1182 RSC.
- I. A. Witońska, M. J. Walock, M. Binczarski, M. Lesiak, A. V. Stanishevsky and S. Karski, J. Mol. Catal. A: Chem., 2014, 393, 248–256 CrossRef.
- R. Baran, I. I. Kamińska, A. Śrebowata and S. Dzwigaj, Microporous Mesoporous Mater., 2013, 169, 120–127 CrossRef CAS.
- J. Zhou, Y. X. Han, W. J. Wang, Z. Y. Xu, H. Q. Wan, D. Q. Yin, S. R. Zheng and D. Q. Zhu, Appl. Catal., B, 2013, 134–135, 222–230 CrossRef CAS.
- Y. L. Ren, G. Y. Fan, W. D. Jiang, B. Xu and F. Liu, RSC Adv., 2014, 48, 25440–25446 RSC.
- H. Y. Deng, G. Y. Fan, C. Y. Wang and L. Zhang, Catal. Commun., 2014, 46, 219–223 CrossRef CAS.
- S. K. Smart, A. I. Cassady, G. O. Lu and D. J. Martin, Carbon, 2006, 44, 1034–1047 CrossRef CAS.
- D. D. Shao, G. D. Sheng, C. L. Chen, X. K. Wang and M. Nagatsu, Chemosphere, 2010, 79, 679–685 CrossRef CAS PubMed.
- K. Yang and B. S. Xing, Environ. Pollut., 2007, 145, 529–537 CrossRef CAS PubMed.
- P. Singh, M. V. Kulkarni, S. P. Gokhale, S. H. Chikkali and C. V. Kulkarni, Appl. Surf. Sci., 2012, 258, 3405–3409 CrossRef CAS.
- I. Lopez-Corral, B. Irigoyen and A. Juan, Hydrogen energy, 2014, 39, 8780–8790 CrossRef CAS.
- R. Oriňáková and A. Oriňák, Fuel, 2011, 90, 3123–3140 CrossRef.
- C. Antonetti, M. Oubenali, A. M. R. Galletti, P. Serp and G. Vannucci, Appl. Catal., A, 2012, 421–422, 99–107 CrossRef CAS.
- M. V. Landau, S. V. Savilov, M. N. Kirikov, N. B. Cherkasov, A. S. Ivanov, V. V. Lunin, Y. Koltypinc and A. Gedanken, Mendeleev Commun., 2011, 21, 125–128 CrossRef CAS.
- P. D. Zgolicz, J. P. Stassi, M. J. Yañez, O. A. Scelza and S. R. Miguel, J. Catal., 2012, 290, 37–54 CrossRef CAS.
- L. C. Jiang, H. Z. Gu, X. Z. Xu and X. H. Yan, J. Mol. Catal. A: Chem., 2009, 310, 144–149 CrossRef CAS.
- H. Vu, F. Goncalves, R. Philippe, E. Lamouroux, M. Corrias, Y. Kihn, D. Plee, P. Kalck and P. Serp, J. Catal., 2006, 240, 18–22 CrossRef CAS.
- X. X. Ma, Y. Liu, X. Q. Li, J. G. Xu, G. D. Gu and C. H. Xia, Appl. Catal., B, 2015, 165, 351–359 CrossRef CAS.
- C. H. Xia, X. X. Ma, S. J. Liu and P. Fan, Procedia Environ. Sci., 2012, 16, 289–292 CrossRef CAS.
- C. H. Xia, Y. Liu, S. W. Zhou, C. Y. Yang, S. J. Liu, S. Z. Guo, Q. Liu, J. B. Yu and J. P. Chen, Catal. Commun., 2009, 10, 1443–1445 CrossRef CAS.
- C. H. Xia, Y. Liu, S. W. Zhou, C. Y. Yang, S. J. Liu, J. Xu, J. B. Yu, J. P. Chen and X. M. Liang, J. Hazard. Mater., 2009, 16, 1029–1033 CrossRef PubMed.
- A. Y. Cao, C. L. Xu, J. Liang, D. H. Wu and B. Q. Wei, Chem. Phys. Lett., 2001, 344, 13–17 CrossRef CAS.
- P. Singh, M. V. Kulkarni, S. P. Gokhale, S. H. Chikkali and C. V. Kulkarni, Appl. Surf. Sci., 2012, 258, 3405–3409 CrossRef CAS.
- Y. C. Xing, J. Phys. Chem. B, 2004, 108, 19255–19259 CrossRef CAS.
- M. A. Aramendía, V. Borau, C. Jiménez, J. M. Marinas and A. Moreno, Colloids Surf., A, 1996, 106, 161–165 CrossRef.
- K. Seiichi, I. Dayu and A. Kaoruo, Adsorption science [M], Chemical Industry Press, Beijing, 2006, pp. 84–88 Search PubMed.
- Z. G. Zhao, Application principle of adsorption [M], Chemical Industry Press, Beijing, 2005, pp. 70–78 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21213a |
| This journal is © The Royal Society of Chemistry 2016 |