Initiation of anionic polymerization of acrylonitrile with tertiary amines and ethylene or propylene oxide: some mechanistic aspects
Yakov I. Estrin,
Alexander E. Tarasov *,
Alexander A. Grishchuk,
Alexander V. Chernyak and
Elmira R. Badamshina*
*,
Alexander A. Grishchuk,
Alexander V. Chernyak and
Elmira R. Badamshina*
Institute of Problems of Chemical Physics of RAS, 1, Academician Semenov Ave, Chernogolovka, 142432, Russia. E-mail: badamsh@icp.ac.ru; Fax: +7 4965221770; Tel: +7 4965224476
First published on 20th October 2016
Abstract
The results of the study of the mechanisms of the initiation reactions of anionic polymerization of acrylonitrile under the action of a new initiating system with tertiary amine–ethylene (or propylene) oxide are presented. 1H NMR spectroscopy it was shown that, ethylene oxide slowly and in equilibrium reacts with 1,4-diazabicyclo[2.2.2]octane at room temperature with the formation of zwitterions, which initiate the further polymerization of ethylene oxide. In the presence of acrylonitrile, zwitterions quickly join it causing rapid growth of polyacrylonitrile chains on the formed carbanions. The reaction scheme for the initiation is suggested. The scheme was confirmed by quantum-chemical calculations of thermodynamic parameters for the reversible reactions of addition of ethylene (or propylene) oxide to some tertiary amines with the formation of zwitterions. The relationship between the molecular structure of tertiary amines and their ability to form zwitterions is discussed.
Introduction
Nowadays, industrial-scale production of polyacrylonitrile (PAN) is based on the process of radical polymerization.1,2 But due to the presence of an electron-deficient double bond in its structure, acrylonitrile (AN) is capable of polymerizing not only by a radical mechanism but also through an anionic one. Extensive studies on both mechanisms initiated in the late 50s has gained thousands of publications concerning the radical mechanism, including those published recently,3–6 while those concerning the anionic polymerization of AN are scarce.Meanwhile, the anionic polymerization of AN has several advantages compared to the radical one, such as the possibility of conducting the polymerization with a high rate at low temperatures (from room to cryogenic ones) with high yields of the polymer. Moreover, some researchers hoped to carry out anionic polymerization of AN according to the principle of living polymerization with obtaining PAN with a narrow molecular weight distribution which could be regulated by varying the monomer/initiator ratio.
In the first studies in the field, anionic polymerization of AN was initiated with tertiary phosphines,7,8 charge-transfer complexes such as sodium anthracene, disodium anthracene, lithium anthracene, lithium naphthalene, and sodium naphthalene,9 organometallic compounds of alkali metals (largely Li),10–13 allyl complexes of group IV metals,14 and phosphites.15–17 In the case of phosphites, the polymerization is carried out in the presence of activators—metal alcoholates or tetra alkyl ammonium bromides—that are needed to ensure the fabrication of high-molecular weight products. For this reason, the thus prepared polymers may contain metallic impurities. Recently,18 highly isotactic PAN was obtained by precipitation polymerization in xylene under the action of dibutylmagnesium.
In contrast to tertiary phosphines, tertiary amines do not initiate the polymerization of AN. With only the exception of 1,4-diazabicyclo[2.2.2]octane (DABCO) whose complexes with some glycidyl ethers and glycidyl esters were found to initiate AN polymerization yielding oligomeric PAN (Mn = 3700–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800);19 in this case, the polymerization also did not occur in the absence of epoxides.
800);19 in this case, the polymerization also did not occur in the absence of epoxides.
Previously,20 we have found that the anionic polymerization of AN in solution can be initiated by the products of interaction between ethylene oxide (EO) or propylene oxide (PO) and bicyclic tertiary amines at temperatures below 20 °C. In contrast to other initiators of AN polymerization,10–18 our initiators contain no metal atoms and no elements heavier than an O atom, which is attractive for the synthesis of high-purity PAN for production of high quality carbon fibers. The polymerization rate can be regulated by variations in temperature and solvent composition.
In this communication, we report the results of our more detailed NMR study (supported by quantum-chemical calculations) on the mechanism of initiation, that is, on the formation of active centers during anionic polymerization of AN in the presence of new initiating systems such as DABCO–EO.
Experimental
Materials
Acrylonitrile (AN, Acros Organics, 99+%) was dried over freshly calcined CaCl2 and then vacuum-distilled into a cell with CaH2. Dimethylsulfoxide-d6 (DMSO-d6, 99.8% pure) was held over CaH2, distilled in vacuum (62 °C/4 mm Hg), and stored under dry argon. Before use, 1,4-diazabicyclo[2.2.2]octane (DABCO, Acros Organics, 97%) was additionally dried by thermo-vacuum treatment. Ethylene oxide (EO, Fluka, purum, 99.8%) was distilled in vacuum and stored over CaH2. Polyethylene glycol-400 (PEG-400, Merck, Germany) was used as purchased. Argon gas (Linde Gas Rus, 99.998%) was used without further purification.Synthesis and characterization
A weighed amount of DABCO was vacuum-treated to eliminate moisture in a round-bottom vessel attached to a vacuum line. To this vessel, DMSO-d6 (solvent) and EO were added via distillation in vacuum; the vessel was filled with Ar and allowed to warm up to room temperature. The mixture was mixed with a magnetic stirrer for 24 h, after which the samples for NMR characterization were taken. AN–DABCO–EO mixtures were prepared from individual solutions of the components in DMSO-d6 just before analysis.1H NMR spectra were taken with an AVANCE III spectrometer (Bruker, 500 MHz) by using TMS as a reference sample and DMSO-d6 as a solvent. The concentration of reagents in DMSO-d6 was around 10 wt%.
The rates of heat release during polymerization were recorded at 25 °C with a standard Calvet calorimeter.
Quantum-chemical calculations
The molecular geometry was optimized in terms of DFT theory in the M06-2X/6-311G** approximation,21 using the program package Gaussian 09.22 The effect of the solvent was taken into account by using the model of polarization continuum.23 The nature of the steady points was established based on the calculated frequencies of normal vibrations (matrix of Gesse force constants). The enthalpy H and Gibbs free energy G were calculated for T = 298 K and then used to obtain the enthalpy change ΔH and free energy change ΔG in reaction. Equilibrium constants were calculated from the expression ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = −ΔG/RT.
k = −ΔG/RT.
Results and discussion
Experimental results
According to ref. 20, polymerization of AN can only be initiated by bicyclic amines in combination with olefin oxides. Interaction of EO with a bicyclic amine (e.g. DABCO) is assumed to result in the opening of the epoxy cycle and formation of a 2-oxyethyl-tetraalkyl ammonium zwitterion in a reversible reaction in accordance with Scheme 1.The inertness of acyclic amines in this reaction was associated with the steric hindrance for the tunnelling transition of an N atom through the NR1R2R3 plane as shown in Scheme 2. As a result, the equilibrium shifts to the right, which makes the formation of zwitterions thermodynamically unfavorable.
In order to shed new light on the mechanism for the formation of active species and initiation of polymerization, we explored the processes taking place during the interaction of tertiary amine with epoxide using the technique of 1H NMR spectroscopy.
For the first time, the polymerization of EO under the action of trimethylamine (TMA) was reported in ref. 24. Interaction of tertiary amines with EO leads to formation of poly(ethylene oxide) (PEO) due to chain growth on zwitterions (Scheme 3) with the example of DABCO as the tertiary amine.
Fig. 1a–c present the 1H NMR spectra of the reaction products formed at DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1
50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 1
10, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively, which we performed in our work. In all cases, the signals’ positions are the same, their intensity only varies.
2, respectively, which we performed in our work. In all cases, the signals’ positions are the same, their intensity only varies.
According to ref. 25 signals at 3.02 and 3.36 ppm were assigned to the CH2 group in the DABCO–EO active center. The signal at 2.98 ppm also belongs to the CH2 group in the active center DABCO–nEO, it slightly shifted because of the different length of the EO chain linked with DABCO. Signals at 3.40–3.61 ppm are from the CH2 groups of the polyEO main chain while those at 3.96–4.18 and 6.50 ppm are from terminal CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– groups.26 The spectra contain no signals corresponding to the addition of two EO molecules to the opposite nitrogen atoms in DABCO to yield a double-charged tricyclic cage.27
CH– groups.26 The spectra contain no signals corresponding to the addition of two EO molecules to the opposite nitrogen atoms in DABCO to yield a double-charged tricyclic cage.27
Besides the signals from PEO, the NMR spectra are also seen to contain signals from the starting reagents, CH2 groups of ionized DABCO, and also from terminal hydroxy and vinyloxy groups of PEO. The low polymerization rate of EO (the reaction remained incomplete in 24 h) and high amount of unreacted DABCO even at DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (Fig. 1c) are indicative of the equilibrium character of the reaction and its slow initiation.
50 (Fig. 1c) are indicative of the equilibrium character of the reaction and its slow initiation.
It can be assumed that the formation of terminal vinyloxy groups takes place by the following mechanism of chain transfer through the formation of the anion CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–O− (Scheme 4).
CH–O− (Scheme 4).
Furthermore, the anion CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–O− is capable of initiating the growth of a new PEO chain with terminal vinyloxy groups that are not linked with a DABCO molecule.
CH–O− is capable of initiating the growth of a new PEO chain with terminal vinyloxy groups that are not linked with a DABCO molecule.
Such a scheme was suggested to rationalize chain propagation in the anionic polymerization of propylene oxide with the formation of unsaturated terminal groups.28
The number of the formed OH groups (Scheme 4) must be equal to that of vinyloxy groups. But in reality in accordance with the NMR spectra the concentration of OH groups markedly exceeds that of vinyloxy groups. This may be due to chain termination on water molecules present in trace amounts (Scheme 5).
Hence, DABCO initiates the anionic polymerization of EO accompanied by the side reactions of chain transfer and chain termination. A relative intensity of signals from the links in the main PEO chain and from terminal vinyl and hydroxyl groups was used to evaluate the extent of polymerization (η) for PEO. It has been found that η = 4–7 in the case of DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, η = 44–77 for DABCO/EO = 1
2, η = 44–77 for DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and η = 36–45 for DABCO/EO = 1
10, and η = 36–45 for DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Since η is defined by the ratio of chain growth rate to chain termination one, it follows (in view of incomplete DABCO consumption) that, already at DABCO/EO = 1
50. Since η is defined by the ratio of chain growth rate to chain termination one, it follows (in view of incomplete DABCO consumption) that, already at DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 44–77 molecules of EO are consumed for one DABCO molecule; while at DABCO/EO = 1
2, 44–77 molecules of EO are consumed for one DABCO molecule; while at DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 140–170 EO molecules.
50, 140–170 EO molecules.
Therefore, the NMR results confirm that the equilibrium in Scheme 1 is strongly shifted to the left (toward the starting reagents); nevertheless, the newly formed oxonium ion is capable of attaching an EO molecule, thus initiating growth of the PEO chain (Scheme 6).
Since at room temperature the anionic polymerization of EO is rather slow,29 the complete consumption of EO in 10% DMSO solution (at DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) will take several hours. Under similar conditions in THF, a 25% extent of polymerization was attained in 24 h.
2) will take several hours. Under similar conditions in THF, a 25% extent of polymerization was attained in 24 h.
Fig. 2 shows the calorimetric results for the rate of heat release q and EO concentration [EO] as a function of polymerization time t as obtained at DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22, [DABCO]0 = 0.18 M, and [EO]0 = 3.94 M. It follows that, even at exceedingly heigh [EO] values (two orders of magnitude bigger than usually used for initiation of anionic polymerization of AN), EO is consumed in several hours. At these values, the extent of DABCO consumption is low (Fig. 1c). This is just another piece of evidence for the slow initiation and autocatalytic character of the polymerization under consideration. It should be noted that DABCO does not react with AN in the absence of EO, at least for several days. However, upon addition of EO, the polymerization of AN gets started rather soon.
22, [DABCO]0 = 0.18 M, and [EO]0 = 3.94 M. It follows that, even at exceedingly heigh [EO] values (two orders of magnitude bigger than usually used for initiation of anionic polymerization of AN), EO is consumed in several hours. At these values, the extent of DABCO consumption is low (Fig. 1c). This is just another piece of evidence for the slow initiation and autocatalytic character of the polymerization under consideration. It should be noted that DABCO does not react with AN in the absence of EO, at least for several days. However, upon addition of EO, the polymerization of AN gets started rather soon.
 | ||
Fig. 2 Rate of heat release q and EO concentration [EO] as a function of polymerization time t: DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22, [DABCO]0 = 0.18 M, [EO]0 = 3.94 M. 22, [DABCO]0 = 0.18 M, [EO]0 = 3.94 M. | ||
In order to track the process of polymerization initiation, we took a series of 1H NMR spectra of the reaction products formed in reaction mixtures with DABCO/EO/AN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 at different AN conversions (Fig. 3a–c). In this mixture, [AN] was made rather low in order that the concentration of rising PAN was comparable with that of other reagents.
8 at different AN conversions (Fig. 3a–c). In this mixture, [AN] was made rather low in order that the concentration of rising PAN was comparable with that of other reagents.
Signals in the range 5.98–6.04 and 6.22–6.39 ppm were assigned to the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) and
and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 groups of the AN monomer while those at 3.14 and 2.03 ppm, to the –CH– and –CH2– groups of PAN. The position of the other signals is the same as in Fig. 1a–c.
CH2 groups of the AN monomer while those at 3.14 and 2.03 ppm, to the –CH– and –CH2– groups of PAN. The position of the other signals is the same as in Fig. 1a–c.
As follows from Fig. 3a, about 1% DABCO gets ionized during the first minutes after intermixing, whereas the PAN signals are still absent. At a 16% conversion of AN (Fig. 3b), the yield of ionized DABCO is around 16%. In this case, each ionized DABCO molecule gives rise to 27–29 monomer units in oligomeric chains. At 2 h (Fig. 3c), the conversion of AN attained a value of around 90%. At this value, the amount of ionized DABCO molecules increased up to ∼21%. The gradual increase in the number of ionized DABCO molecules with the increasing extent of AN conversion is another indication of slow initiation; in other words, the polymerization rate is higher than the rate of DABCO consumption in the reaction yielding active centers of polymerization. The rate of AN polymerization is higher than that of EO since the signals from the EO homopolymer (3.51 ppm) do not manifest themselves until complete exhaustion of AN (Fig. 3c), although at the end of AN polymerization [EO] is higher than [AN] by a factor of ten (Fig. 3c). Apparently, EO cannot compete with AN for addition to arising zwitterions and growing PAN chains.
As is known, AN is capable of undergoing fast polymerization under the action of anionic initiators even at −50 °C,30 which opens up a way to the preparation of high-molecular weight PAN and its co-polymers with acrylates suitable for fabricating fibers. At this temperature, the initiation efficiency (the initiator fraction spent on the formation of active centers) is below unity, which is typical of slow initiation.
Therefore, the process of slow initiation via formation of zwitterions followed by rapid growth of PAN chains in the DABCO–EO–AN system can be described by Scheme 7.
 | ||
| Scheme 7 Mechanism of the anionic polymerization of acrylonitrile under the action of a bicyclic tertiary amine and ethylene oxide. | ||
The validity of Scheme 7 was confirmed by the results of the quantum-chemical calculations presented in the next section.
Quantum-chemical calculations
In order to estimate the rate of initiation reactions, we have to calculate at least an order of magnitude for the equilibrium constants for the first stages of Scheme 7. Since for the addition of EO to DABCO keq ≪ 1, the direct measurement of keq seems problematic. Moreover, the reaction does not stop at the stage of zwitterion formation; it is followed by the polymerization of EO, so that the equilibrium concentration of zwitterions cannot be attained in principle.In this context, we carried out quantum-chemical calculations for the thermodynamic parameter of the above equilibrium for a series of bicyclic amines used in ref. 20 as well as for some acyclic amines, and the products of their interaction with EO or with AN. For the sake of comparison, the thermodynamic parameters for the reaction of DABCO with PO were also calculated which also initiates the polymerization of AN but at a lower rate.20 The results are collected in Table 1.
| No | Reactive system | Reaction products | ΔH, kJ mol−1 | ΔG, kJ mol−1 | keq 298 K, L mol−1 |
|---|---|---|---|---|---|
| a The superscript shows the number of the N atom in the cycles of compounds 8–13.b The methyl group is connected to the carbon atom located next to the zwitterion oxygen atom (C1) for DABCO–PO.c The methyl group is connected to the carbon atom that is located next to the zwitterion nitrogen atom (C2) for DABCO–PO.d Results obtained for tricyclic diamine (compound 12) in THF were recalculated for DMSO as a solvent; in contrast to compound 13, the structure was optimized as tricyclic diamine. | |||||
| 1 | DABCO–EO in THF |  |
3.65 | 56.48 | 9.81 × 10−11 |
| DABCO–EO in DMSO | −7.94 | 49.41 | 1.75 × 10−9 | ||
| 2 | TEA–EO in THF | 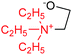 |
26.76 | 88.18 | 2.86 × 10−15 |
| TEA–EO in DMSO | 15.96 | 76.97 | 3.55 × 10−14 | ||
| 3 | TMA–EO in THF |  |
9.82 | 66.24 | 4.08 × 10−11 |
| TMA–EO in DMSO | −0.45 | 55.85 | 1.62 × 10−10 | ||
| 4 | (DABCO–EO) + AN in DMSO |  |
−47.27 | 3.00 | 0.39 |
| 5 | (DABCO–EO–AN) + AN in DMSO | 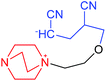 |
−84.69 | −30.53 | 2.57 × 104 |
| 6 | (DABCO–EO) + EO in DMSO | 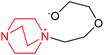 |
−101.7 | −47.40 | 1.92 × 108 |
| 7 | (DABCO–EO) + EO sim in DMSO | 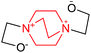 |
17.15 | 73.36 | 1.00 × 10−13 |
| 8 | DABN-N1–EO in DMSOa | 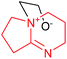 |
156.4 | 216.9 | 1.05 × 10−38 |
| 9 | DABU-N1–EO in DMSO | 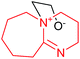 |
64.7 | 255.8 | 1.61 × 10−46 |
| 10 | DABN-N5–EO in THF | 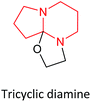 |
−117.4 | −55.3 | 4.9 × 109 |
| 11 | DABN-N5–EO in DMSO |  |
−35.4 | 19.0 | 3.7 × 10−4 |
| 12 | DABU-N8–EO in THF |  |
−106.3 | −43.7 | 4.5 × 107 |
| 13 | DABU-N8–EO in DMSO |  |
−46.2 | 27.7 | 1.4 × 10−5 |
| 14 | DABCO–PO-C1 in DMSOb |  |
28.72 | 87.0 | 5.62 × 10−16 |
| 15 | DABCO–PO-C2/DMSOc |  |
−5.4 | 53.0 | 5.16 × 10−10 |
| 16 | DABU-N8–EO in THF → DMSOd |  |
−114.4 | ||
It follows that the DABCO–EO reaction (number 1 in Table 1) is slightly endothermic in THF and exothermic in DMSO. Calculations show that a negative charge of the O atom in the zwitterion (−0.365, here and below fractional electric charges are given in electron charge units) taking part in the initiation of polymerization is greater than that in the starting EO molecule (−0.178). This is indicative of some redistribution of electron density over the system of σ-bonds (negative induction effect). The atomic charges in the zwitterion are not affected by the solvent type.
The reaction of EO with triethylamine (TEA, number 2) is strongly endothermic in both THF and DMSO. In contrast to the DABCO–EO system, the negative induction effect in the TEA–EO system is less pronounced: the negative charge of the O atom in the zwitterion is −0.354.
Interesting results were obtained for the values of ΔG and keq in the reactions similar to those in Scheme 6. In all cases, the equilibrium is strongly left-shifted, so that the concentration of the zwitterions, in the absence of monomer, is rather low. The largest keq values are observed for the reaction of DABCO; however, unexpectedly close values were found for acyclic TMA (number 3). The reaction of TMA in DMSO turned thermally neutral, but the keq value is only one order of magnitude smaller than in the case of DABCO–EO reaction. Close negative charges of the O atom are observed in both the DABCO–EO and TMA–EO zwitterions. Apparently this is why Staudinger and Lohmann could obtain EO oligomers under the action of TMA,24 although even in the presence of 5% TMA the polymerization lasts for 1–2 weeks at 20 °C. Table 1 also presents the results for two bicyclic amines that initiate the polymerization of AN in the presence of EO:20 1,8-diazabicyclo(5,4,0)undec-7-ene (DABU) and 1,5-diazabicyclo(4,3,0)non-5-ene (DABN), numbers 9–13 in Table 1.
As stated in the previous section, even at DABCO/EO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 the formation of PEO (in the absence of AN) proceeds and the degree of polymerization is DP ≫ 2.0. This implies that the addition of EO molecules to the zwitterions and then to growing PEO chains is faster than the formation of zwitterions and supposedly with higher heat release. Indeed, reaction 6 (Table 1) was found to be highly caloric: the value of ΔH = −101.3 kJ mol−1 actually coincides with the reported value for the thermal effect of EO polymerization (94.5–102) kJ mol−1.31,32
2 the formation of PEO (in the absence of AN) proceeds and the degree of polymerization is DP ≫ 2.0. This implies that the addition of EO molecules to the zwitterions and then to growing PEO chains is faster than the formation of zwitterions and supposedly with higher heat release. Indeed, reaction 6 (Table 1) was found to be highly caloric: the value of ΔH = −101.3 kJ mol−1 actually coincides with the reported value for the thermal effect of EO polymerization (94.5–102) kJ mol−1.31,32
For reaction 4 and 5 (Table 1), the calculated values of ΔH for the addition of the first and second AN molecule had a value of −47.3 and −84.7 kJ mol−1, respectively. The latter is close to the reported magnitudes of −74.5,1 and −76.5 kJ mol−1.33
Our results can also explain the different reactivity of PO and EO. In the case of the addition of PO with the methyl group in position 1 (–N+–CH(CH3)CH2–O–) to the N atom in DABCO the reaction is endothermic (number 14 in Table 1). But in the case of the addition of PO with the methyl group in position 2 (–N+–CH2CH(CH3)–O–) the reaction is slightly exothermic (number 15 in Table 1), and keq is about 30 times lower than in the DABCO–EO system. In this case, the negative charge of the O atom (−0.372) is higher. Upon attachment of the methyl group in position 1 (number 14 in Table 1), the negative charge of the O atom is practically the same in both systems (the positive induction effect caused by the presence of the CH3 group).
Previously,20 we assumed that the role of active centers in the initiation of AN polymerization might be played by N atoms at the cycle vertex. But it turns out that the addition of EO to the vertex N atom in DABN-1-N1 and DABU-1-N1 (number 8 and 9) is less likely than that to the out-of-cycle vertex N atoms in DABN-N5 and DABU-N8 (number 10–13). In this case, we obtain either stable tricyclic products (number 10 and 12) or less stable zwitterions (number 11 and 13).
Our results also give grounds to assume that the reactivity of bicyclic amines in the initiation of AN polymerization in the presence of EO can depend on steric factors. In contrast to DABCO, nitrogen atoms at the vertexes of DABU and DABN bicycles are not out-of-plane, which imposes steric hindrance for free rotation of the –C2H4O− group without overlapping between the van der Waals radii of oxygen and –C–H groups; and this circumstance markedly affects the thermodynamics of zwitterion formation. Moreover, large non-planar cycles in DABU and DABN impose additional steric hindrance for free rotation of –C2H4O− group.
Fig. 4 presents the ball models of zwitterions formed upon addition of an EO molecule to DABCO (Fig. 4a), to TMA (Fig. 4b), to DABN-N1 (Fig. 4c), to DABN-N5 (Fig. 4d), and to DABU-N8 (Fig. 4e). It is clearly seen that the –C2H4O– fragment of the zwitterion can freely rotate around the C–N bond only in the case of DABCO. Apparently, the methyl groups in TMA are also incapable of creating noticeable steric hindrance to the rotation of the –C2H4O– fragment in the zwitterion around the N–C bond, but they can rotate in such a way that their hydrogen atoms will be oriented “vertically” and thus cross the path of the –C2H4O– fragment. Since large cycles in DABN and DABU are non-planar, hydrogen atoms in the CH2 groups adjacent to the vertex N atom restrict the rotation of the –C2H4O– fragment attached to the N1 atoms. A similar restriction also takes place in compounds 4d and 4e (addition of EO to N5 and N8 atoms). In the case of DABN and DABU in THF (number 10 and 12), the third cycle is closed through rupture of the double bond in the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– group, the thermal effect of the reaction being close to that of double bond rupture during polymerization of olefins or opening of the oxide cycle in polymerization of olefin oxides.
C– group, the thermal effect of the reaction being close to that of double bond rupture during polymerization of olefins or opening of the oxide cycle in polymerization of olefin oxides.
 | ||
| Fig. 4 Rotation of the –C2H4O– group around the N–C bond in the products of addition of an EO molecule to DABCO (a), TMA (b), DABN-N1 (c), DABN-N5 (d), and DABU-N8 (e). | ||
In DMSO, cycle closure does not occur; instead, we observe the formation of a zwitterion with a “carbocation” as a counter ion for the carboxy anion; at this situation, the thermal effect of reaction is markedly smaller then in THF (number 11 and 13) but higher than for the EO–DABCO reaction in DMSO. Meanwhile, if we place a tricyclic product optimized in THF into DMSO medium, we will observe no reverse conversion to a zwitterion; it will retain its tricyclic structure, at high ΔH (number 16 in Table 1).
It may be assumed that in DMSO the zwitterions are present in their metastable state. The optimization of the structure in DMSO is interrupted in a local minimum with a high potential barrier, as illustrated in Scheme 8. Therefore, a most probable mechanism for the initiation of AN polymerization in the DABCO–EO system can be described in terms of Scheme 7 corresponding to low initiation efficiency. Given that ΔG for the first AN addition to the initiating zwitterion is close to zero (∼3 kJ mol−1), the second stage in Scheme 7 must be in equilibrium with keq ≈ 1.0. And only the addition of the second, third, etc. AN molecules proceed with ΔH = −84.7 kJ mol−1 and ΔG = −30.6 kJ mol−1. At the third stage, the active center (carbanion) becomes thermodynamically stable and ensures rapid chain propagation.
In DMSO, the behavior of DABU and DABN looks reasonable: the metastable intermediates maybe sufficiently long-living for ensuring the addition of monomer molecules and stabilization of the growing carbanions. But in THF such a situation is unlikely, in view of exothermic formation of the tricyclic structure without the formation of an oxonium–ammonium zwitterion. Nevertheless, both compounds in combination with EO are known to initiate the polymerization of AN in both DMSO and THF.20 These observations allow us to assume that the mechanism of initiation maybe more complicated than that shown in Scheme 7. It cannot also be excluded that this reaction also involves the interaction with amine–EO–AN complexes. This assumption can be checked by calculating potential energy surfaces for the formation of such complexes from starting compounds or for the decomposition of amine–EO–AN compounds (such as number 4 in Table 1).
The possibility for the addition of EO to both N atoms in DABCO to yield a symmetric bis-zwitterion (Scheme 9) was also discussed in ref. 20. The reaction was found to be endothermic even in DMSO and the constant of second equilibrium was smaller than the first one by four orders of magnitude. Accordingly, such reaction (number 7 in Table 1) is hardly probable because of Coulombic repulsion of the two parallel dipoles. This is in line with the NMR spectra in Fig. 1a–c: no double charged DABCO cages were detected.
Concluding remarks
In the DABCO–EO system, slow reversible reaction of EO addition to the N atom in DABCO yielding a zwitterion is followed by relatively fast addition of EO to the zwitterion thus stabilizing the latter due to the formation of a covalent C–N bond. Our observations that (i) the reaction of zwitterion formation consumes only about 21% DABCO (event at a large excess of EO) and (ii) synthesized PEO exhibits a high polymerization degree imply a low rate for the initiation reaction and its low efficiency. But upon addition of relative small amounts of monomeric AN to the above system, its polymerization proceeds rapidly, despite the fact that the initiation rate remains practically the same.DFT calculations in the M062X/6-311g** approximation supported the above reaction scheme (see Scheme 7). For AN polymerization under the action of the DABCO–EO system, the observed difference between the reaction rates in THF and DMSO agrees with the difference between the respective equilibrium constants (18-fold). Calculated data also explain the differences between the reactivity of tertiary amines in initiating AN polymerization with epoxides by the specificity of their spatial configuration.
The polymerization of AN initiated by the DABCO–EO system gets rapidly decelerated well in advance of complete consumption of the monomer due to the termination of growing chains complicated by the reactions of chain transfer. Such reactions not only affect the polymerization degree but also result in formation of branched macromolecules. These aspects of the problem deserve further investigation.
Our results suggest that not only DABCO but also some tertiary amines (such as DABN, DABU, and TMA) are capable of initiating the polymerization of AN in the presence of epoxides. But in contrast to bicyclic amines with large non-planar cycles imposing steric hindrance for the addition of epoxides, symmetric DABCO molecules generate no difficulties. Being the most reactive reagent, DABCO is an accessible and inexpensive chemical that is convenient for use in industrial-scale production of high-purity PAN by anionic polymerization.
Acknowledgements
The reported study was partially supported by RFBR, research project No 15-03-04592. The authors are grateful also to Sergei Karpov for the help in carrying out quantum-chemical calculations and Yury Scheck for the translation of the article.References
- M. M. Wu, in Encyclopedia of Polymer Science and Technology, ed. H. F. Mark, Wiley, New York, 2004, vol. 1, p. 124 Search PubMed
.
- Wikipedia, https://en.wikipedia.org/wiki/Polyacrylonitrile, accessed May 2016.
- Y. Xu, J. Sun, H. Chen and L. Bai, RSC Adv., 2015, 5, 58393 RSC
.
- Y. Xu, J. Sun, H. Chen and L. Bai, RSC Adv., 2015, 5, 37780 RSC
.
- A. V. Shlyahtin, I. E. Nifant’ev, V. V. Bagrov, D. A. Lemenovskii, A. N. Tavtorkin and P. S. Timashev, Green Chem., 2014, 16, 1344 RSC
.
- A. Ju, Y. Yan, D. Wang, J. Luo, M. Ge and M. Li, RSC Adv., 2014, 4, 64043 RSC
.
- L. Horner, H. Jurgeleit and K. Klüpfel, Liebigs Ann. Chem., 1955, 591, 108 CrossRef CAS
.
- V. Jaaks, C. D. Eisenbach and W. Kern, Makromol. Chem., 1972, 161, 139 CrossRef
.
- A. Zilkha and Y. Avny, J. Polym. Sci., Part A: Gen. Pap., 1963, 1, 549 CrossRef CAS
.
- B. L. Erussalimsky and A. V. Novoselova, Acta Polym., 1975, 26, 293 Search PubMed
.
- A. V. Novoselova, G. A. Orlova, B. L. Erussalimsky, H.-J. Adler and W. Berger, Acta Polym., 1985, 36, 599 CrossRef CAS
.
- A. Novoselova, V. Zgonnik, Y. Sazanov, G. Lyubimova, G. Orlova and N. Shirokov, Vysokomol. Soedin., Ser. A, 1992, 34, 59 CAS
; A. Novoselova, V. Zgonnik, T. Spirina, G. Lyubimova, G. Orlova and N. Shulagina, Vysokomol. Soedin., Ser. A Ser. B, 1993, 35, 510 Search PubMed
.
- A. V. Novoselova, V. V. Shamanin and L. V. Vinogradova, Polym. Sci., Ser. B, 2009, 51, 205 CrossRef
.
- B. L. Erussalimsky and W. Berger, Acta Polym., 1988, 39, 632 CrossRef
; B. L. Erussalimsky and L. A. Fedorova, Acta Polym., 1988, 39, 667 CrossRef
.
- T. Ogawa and P. Quintana, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 2517 CrossRef CAS
.
- T. Ogawa and J. Romero, Eur. Polym. J., 1977, 13, 419 CrossRef CAS
.
- N. Terán, M. R. Estrada and T. Ogawa, J. Macromol. Sci., Part A: Pure Appl. Chem., 2005, 12, 1679 CrossRef
.
- F. Zhang, Z.-X. Xu and R.-G. Jin, Acta Polym. Sin., 2008, 1, 1102 Search PubMed
.
- I. Ikeda, S. Mitsumoto, Y. Yamada and K. Suzuki, Polym. Int., 1991, 26, 115 CrossRef CAS
.
- Y. Estrin, A. Grishchuk, A. Tarasov, E. Perepelitsina and E. Badamshina, Polym. Sci., Ser. B, 2016, 58, 19 CrossRef CAS
.
- A. J. Cohen, P. Mori-Sanchez and W. Yang, Chem. Rev., 2012, 112, 289 CrossRef CAS PubMed
.
- R. Dennington, T. Keith and J. Millam, GaussView, Gaussian 09: ES64L-G09RevD.01 24-Apr-2013, Semichem Inc., Shawnee Mission, KS, 2009 Search PubMed
.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999 CrossRef CAS PubMed
.
- H. Staudinger and H. Lohmann, Liebigs Ann. Chem., 1933, 505, 41 CrossRef CAS
.
- W. Clegg, B. Golding, R. Harrington and R. Scott, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2004, 60, 291 Search PubMed
.
- H. J. Reich, Proton Chemical Shifts, 2016, http://www.chem.wisc.edu/areas/reich/handouts/nmr-h/hdata.htm Search PubMed
.
- Ü. H. Yildiz, K. Koynov and F. Gröhn, Macromol. Chem. Phys., 2009, 210, 1678 CrossRef
.
- G. Gee, W. C. E. Higginson, K. J. Taylor and M. W. Trenholme, J. Chem. Soc., 1961, 4892 Search PubMed
.
- J. Furukawa and T. Saegusa, Polymerization of Aldehydes and Oxides, Interscience Publishers, A Div. of John Wiley & Sons, N-Y, London, Sydney, 1963 Search PubMed
.
- I. L. Artamonova, A. V. Novoselova, S. I. Vinogradova, L. M. Budanova, L. N. Korzhavin and B. L. Erussalimsky, Acta Polym., 1984, 35, 65 CrossRef CAS
.
- C. T. Moptimer, Reactions Heats and Bond Strengths, Pergamon Press, Oxford, London, New York, Paris, 1962 Search PubMed
.
- K. J. Ivin, in Polymer Handbook, ed. J. Brandrup and E. H. Immergut, Wiley, New York, 1999, p. II/374 Search PubMed
.
- K. J. Ivin, in Polymer Handbook, ed. J. Brandrup and E. H. Immergut, Wiley, New York, 1999, p. II/368 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |










