Controlled synthesis of porous Co3O4 nanofibers by spiral electrospinning and their application for formaldehyde oxidation
Abstract
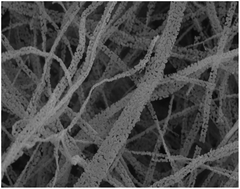
Nanofiber mats have been widely used in various fields owing to their high porosity, high specific area and three-dimensional architecture. Porous cobaltosic oxide (Co3O4) nanofiber mats were mass-produced by spiral electrospinning and controlled calcination, after which the three-dimensional scaffold still existed and consisted of well-twisted continuous nanofibers. The nanofiber was composed of neat Co3O4 nanoparticles with a necklace-like arrangement. The mechanism for the formation of porous Co3O4 necklaces was proposed by investigating the structural evolution of the calcined fibers, their elemental composition, crystal structure and the presence or absence of different functional groups. Moreover, the porous Co3O4 nanofiber mats exhibited high catalytic activity (98 °C, 100% conversion) and catalytic stability (160 h, nearly 100% conversion) for the oxidation of formaldehyde, which was mostly attributed to the increases in the porosity and the specific area.

 Please wait while we load your content...
Please wait while we load your content...