Activation of radical addition to graphene by chemical hydrogenation†
Abstract
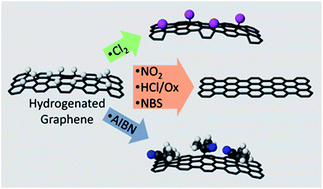
We report several methods of chemical dehydrogenation of hydrogenated graphene (HG), characterizing the results using Raman, X-ray photoelectron spectroscopy, and electrical conductivity measurements. Notably, the hydrogen–graphene bonds appear to activate the graphene toward subsequent reaction such that, in several cases, the addition of the dehydrogenating agent to the graphene accompanies the removal of hydrogen. We compare the uptake of chemical groups on HG to those on pristine graphene and find that HG reacts more readily than pristine graphene with radical generators such as chlorine and AIBN.

 Please wait while we load your content...
Please wait while we load your content...