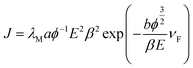Spatially branched CdS–Bi2S3 heteroarchitecture: single step hydrothermal synthesis approach with enhanced field emission performance and highly responsive broadband photodetection†
Prashant K. Bankar‡
ab,
Mahendra S. Pawar‡b,
Amit S. Pawbakebc,
Sambhaji S. Waruled,
Dattatray J. Late*b and
Mahendra A. More*a
aCentre for Advanced Studies in Materials Science and Condensed Matter Physics, Department of Physics, Savitribai Phule Pune University, Pune 411007, India. E-mail: mam@physics.unipune.ac.in
bPhysical and Material Chemistry Division, CSIR – National Chemical Laboratory, Pune, 411008, Maharashtra, India. E-mail: dj.late@ncl.res.in
cSchool of Energy Studies, Department of Physics, Savitribai Phule Pune University, Pune 411007, India
dDepartment of Physics, NowrosjeeWadia College, Savitribai Phule Pune University, Pune- 411001, India
First published on 29th September 2016
Abstract
This report explores the controlled hierarchical synthesis of CdS nanostructure branches on Bi2S3 nanorod cores via a facile single step hydrothermal route. Morphological and structural studies reveal the formation of CdS–Bi2S3 heteroarchitecture with excellent stoichiometry between the constituent elements. The growth of CdS over Bi2S3 strongly depends on optimization of the reaction conditions, especially low PVP concentration. Furthermore, the as-synthesized CdS–Bi2S3 heteroarchitecture demonstrates multifunctionality in field emission and photoresponse. Interestingly, the CdS–Bi2S3 heteroarchitecture shows enhanced field emission properties such as low turn-on field (∼1.8 V μm−1 for 10 μA cm2), high emission current density and better current stability in comparison to Bi2S3 and other nanostructures. The as-synthesized CdS–Bi2S3 heteroarchitecture exhibits considerable response and recovery times, ∼207 ms and 315 ms, respectively in comparison to bare Bi2S3 nanostructures (∼655 ms and 678 ms). The present results demonstrate CdS–Bi2S3 heteroarchitecture as a potential candidate for future optoelectronic device applications.
Introduction
Shape controlled growth of one-dimensional (1D) semiconductor heterostructures through a hierarchical assembly process has attracted significant scientific attention lately, because of the size, shape, and morphology dependent properties of nanomaterials that are key to various applications.1–8 It is further recognized that such interesting properties also depend strongly on the synthesis, processing methods and interfaces at the individual counterparts. Therefore, development of precise heteroarchitecture with well defined morphology remains an important research endeavour. In this context, it is necessary to explore a facile, large scale, low-cost route enabling synthesis of novel heteroarchitectures possessing high crystallinity and clean surfaces. Among the various synthesis methods, hydrothermal routes offer a distinct advantage over the others, because their specific high pressure (superheating) growth character is expected to generate the desired morphologies of the heteroarchitectures in a single step.Although, hydrothermal method has been widely used to synthesize Bi2S3, nanostructures with different morphologies, research on well-defined Bi2S3 heterostructures via the facile hydrothermal approach is sparse. Recently, Panmand and co-workers8 had attempted decoration of CdS nanoparticles on Bi2S3 nanowires using solvothermal route for visible light photo catalyst application. Also, Fang et al.2 have reported photocatalytic activity of CdS nanoparticles on Bi2S3 nanowire obtained by wet chemical method performed at higher temperature.
In the present study, single step hydrothermal approach is effectively used to obtain CdS–Bi2S3 heteroarchitecture possessing unique morphology. Interestingly, the CdS–Bi2S3 heteroarchitecture exhibits enhanced field electron emission (FE) behaviour and photodetection with excellent response in a broadband wavelength region, owing to tuning of the properties of individual counterpart, and high electron mobility.9,10 The superior FE characteristics and photoresponse behaviour is attributed due to enhancement in the aspect ratio due to unique morphology of the heteroarchitecture and modulation of electronic properties at the interface.
Experimental
Materials
Bismuth nitrate (Bi(NO3)3·5H2O), thiourea ((NH3)2CS), cadmium nitrate (Cd(NO3)2·4H2O), and polyvinylpyrrolidone (PVP) all of analytical grades were used without further purification for synthesis of Bi2S3 nanostructures and CdS–Bi2S3 heteroarchitecture.Synthesis method
In a typical hydrothermal synthesis of Bi2S3 nanostructures, Bi (NO3)3·5H2O (0.970 g) and (NH3)2CS (0.6089 g) were dissolved in 80 ml mixture of distilled water (DW) and ethylene glycol (EG) with volume ratio as 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was transferred into 100 ml Teflon-lined autoclave which was kept at 130 °C for 9 h followed by natural cooling to room temperature. The obtained black precipitate then washed with distilled water and absolute ethanol for a number of times, and then kept for drying at 60 °C for 2 h.
1. The mixture was transferred into 100 ml Teflon-lined autoclave which was kept at 130 °C for 9 h followed by natural cooling to room temperature. The obtained black precipitate then washed with distilled water and absolute ethanol for a number of times, and then kept for drying at 60 °C for 2 h.
In the next set of experiments, the synthesis of CdS–Bi2S3 heteroarchitecture was carried out. For this, 0.970 g of Bi(NO3)3·5H2O and 0.6089 g of (NH3)2CS were dissolved in 20 ml EG and 30 ml DW, respectively. In another glass beaker, 0.128 g of Cd(NO3)2 and 0.005 g of PVP were dissolved in 30 ml DW. Both these solutions were mixed and stirred for 20 min. Further, hydrothermal process followed is similar to Bi2S3 nanostructures.
Material characterizations
The structural analysis of the as-synthesized the Bi2S3 nanostructures and CdS–Bi2S3 heteroarchitecture was carried out using an X-ray diffractometer (XRD, D8 advance, Bruker AXS), where as for surface morphological studies, a field-emission scanning electron microscope (FESEM, Hitachi S-4800) was used. Furthermore, structural and morphological investigations were performed using transmission electron microscopy (FEI TITAN 300 KV, equipped with FEG source) and (Technai G2 U20 Twin, FEI) operated in high resolution (HR) mode. The optical properties were investigated by using (UV-visible-NIR spectrophotometer-Model-JASCO 670).The field emission current density versus applied electric field (J–E) and emission current versus time (I–t) characteristics were measured in a planar ‘diode’ configuration in an all-metal vacuum chamber evacuated to base pressure of 1 × 10−8 mbar. In a typical ‘diode’ configuration, a semitransparent cathodoluminescent phosphor screen (diameter ∼ 50 mm) was held parallel to the cathode. For material(s) synthesized in powder form, the cathode is obtained by sprinkling very small quantity of the powder onto a piece of UHV compatible conducting carbon tape (∼0.25 cm2). This piece of carbon tape (with sprinkled powder) was pasted on copper rod connected to a linear motion drive, facilitating variation in cathode–anode separation. The FE measurements were carried out at a constant cathode–anode separation of 2 mm, the emission current was acquired on Keithley electrometer (6514) by varying the applied voltage between the cathode and anode with a step of 40 V (0–40 kV, Spellman, U.S.). The details of vacuum processing and FE measurements are described elsewhere.11
Device fabrication
In device fabrication part we have used ITO (tin doped indium oxide) as substrate and made a 'rectangular' scratch (notch) on the conducting surface. An appropriate amount of Bi2S3 and CdS–Bi2S3 powders were dispersed (separately) into N-methyl-2-pyrrolidone (NMP) followed by ultrasonication for 10 min. Then, the samples subsequently drop casted onto the scratched regions. The devices were dried in vacuum furnace at 120 °C to improve the adhesion of the sample with substrate.12Photodetection
The photodetection experiments were carried out using different LED's such as blue, green, orange, yellow and red. We have also used white light source for the photoresponse study. All the photodetection measurements were carried out at room temperature.Results and discussion
XRD
Fig. 1 depicts a typical XRD pattern of the as-synthesized Bi2S3 nanostructures (black color) and CdS–Bi2S3 heteroarchitecture (red color) obtained under hydrothermal conditions. The XRD patterns exhibit a set of well defined diffraction peaks implying crystalline nature of the as-synthesized products. The observed diffraction peaks were indexed to orthorhombic phase of Bi2S3 (a = 11.11, b = 11.25, and c = 3.91 Å, (JCPDS card no. 75-1306)). The intensities of the diffraction peaks due to CdS are noticed to be relatively weaker than that of Bi2S3. This is due to the variation in amount of materials present in the heterostructure. Interestingly, no diffraction peaks due to other phases or impurities were observed, suggesting formation of pure CdS–Bi2S3 phase under the prevailing experimental conditions. | ||
| Fig. 1 XRD patterns of the Bi2S3 nanoflowers (black color) and CdS–Bi2S3 heteroarchitecture (red color). | ||
Surface morphology
The surface morphology of the as-synthesized Bi2S3 nanostructures and CdS–Bi2S3 heteroarchitecture is depicted in Fig. 2. The Fig. 2(a) shows the FESEM images of the pristine Bi2S3 urchin-like nanoflowers with average size in the range of 1–3 μm. A magnified image (inset of Fig. 2(a)) reveals that the flower is comprised of smooth Bi2S3 nanorods with average length of ∼1 μm and diameter in the range of 50–100 nm. The surface morphology of the CdS–Bi2S3 heteroarchitecture (Fig. 2(b) to (d)) is characterized as presence of pine tree like hierarchical structures. The Bi2S3 nanorods act as a backbone for the growth of CdS nanostructures and the overall heteroarchitecture resembles to the leaves of a pine tree. There are rows of Bi2S3 nanorods and most of the CdS nanostructures grow in the direction perpendicular to the surface of Bi2S3 nanorods. The secondary CdS branches (mostly nanorods) developed on the Bi2S3 nanorods have length of 20–50 nm and diameter in the range of 20–30 nm (Fig. 2(d)). A careful observation of the high magnification image reveals that the CdS nanostructures exhibit four-fold symmetry around the Bi2S3 nanorod axis. The energy dispersive X-ray analysis (EDAX) of as synthesized products is depicted in Fig. S1.† It is worth mentioning here that under the optimized set of process variables, CdS–Bi2S3 heteroarchitecture possessing pine tree leaves like morphology has been obtained via single-step hydrothermal approach.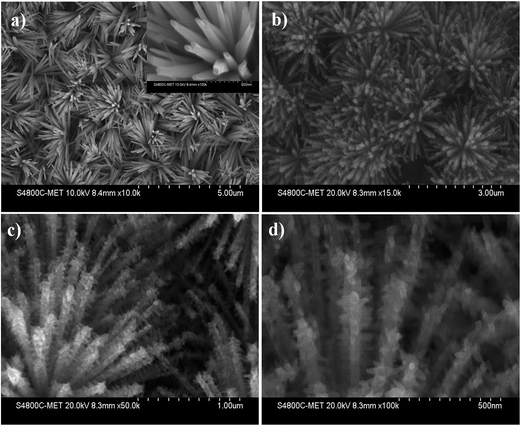 | ||
| Fig. 2 FESEM images of (a) as-synthesized Bi2S3 nanoflowers with high magnification image as inset, and (b–d) CdS–Bi2S3 heteroarchitecture. | ||
The detailed structural and crystalline characteristics of these nanostructures have been carried out with the help of TEM analysis. Fig. 3(a) and (b) shows a typical TEM image of separated Bi2S3 nanorods from the Bi2S3 nanoflowers. The average diameter and length of nanorods are estimated to be 15–20 nm and 1 μm, respectively. The high resolved TEM image (Fig. 3(c)) shows inter-planar distance of ∼0.5 nm, which corresponds to (120) plane of Bi2S3. Furthermore, the selected area electron diffraction pattern (Fig. 3(d)) confirms single crystalline nature of Bi2S3 nanostructures which is in good agreement with the XRD analysis.
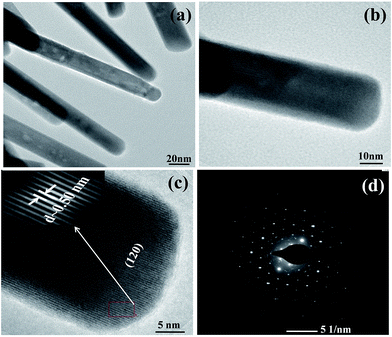 | ||
| Fig. 3 TEM images (a and b) low and high magnification, (c) HRTEM, and (d) SAED pattern of corresponding Bi2S3 nanoflowers. | ||
Fig. 4(a) and (b) show the TEM image of CdS–Bi2S3 heteroarchitecture which clearly reveals the well decoration of CdS nanostructures (branches) on the Bi2S3 nanorods (stem). The length of CdS nanostructures is estimated to be ∼10–15 nm. The TEM results support the FESEM analysis. The Fig. 4(c) depicts HRTEM image of the CdS–Bi2S3 heteroarchitecture consisting of two different lattice spacing (d). The observed ‘d’ values, ∼0.5 and 0.31 nm correspond to the (120) plane of orthorhombic Bi2S3 and (101) plane of hexagonal CdS, respectively. The SAED pattern of CdS–Bi2S3 heteroarchitecture (Fig. 4(d)) reveals its polycrystalline nature. The scanning transmission electron microscope – high angle annular dark field (STEM-HAADF) image, shown in Fig. S2,† indicates the elemental distribution maps of the Bi, S, and Cd, the constituents of CdS–Bi2S3 heteroarchitecture. Furthermore, in order to understand the optical properties of as-synthesized materials, UV-Vis spectra were recorded at room temperature, and shown in Fig. S3.†
 | ||
| Fig. 4 TEM images (a and b) low and high magnification, (c) HRTEM (d) SAED pattern of CdS–Bi2S3 heteroarchitecture. | ||
Field emission
The FE current density versus applied field (J–E) characteristics of the pristine Bi2S3 nanoflowers and CdS–Bi2S3 heteroarchitecture emitters are depicted in Fig. 5(a). Before recording the J–E observations, a pre-conditioning in terms of removal of surface asperities and contaminants by residual gas ion bombardment was carried out. For this, the emitter was kept at 2 kV with respect to the anode for 5 min duration. In the present studies, the applied field (E) is defined as E = V/d, where V is the applied voltage and d is the separation between the emitter and the anode. The emission current density (J) is estimated as J = I/A, where I is the measured value of emission current and A is the actual (total) area of the emitter surface. From the observed J–E characteristics, the values of the turn-on field, defined as field required draw an emission current density of 10 μA cm−2, were found to be 2.9 and 1.8 V μm−1, for the pristine Bi2S3 nanoflower and CdS–Bi2S3 heteroarchitecture emitters, respectively. The emission current density varying rapidly (exponentially) with gradual increments in the applied field indicates that the emission is as per the Fowler–Nordheim theory.13 An emission current density of 181 μA cm−2 has been drawn at an applied field of 4.1 V μm−1 from the pristine Bi2S3 emitter. Interestingly, at relatively lower applied field of 3.1 V μm−1, the CdS–Bi2S3 heteroarchitecture emitter delivers large emission current density of 677 μA cm−2. The present results are compared to the earlier reports on other semiconductor nanostructures and heteroarchitecture emitters, depicted in Table 1.14–20 The dependence of FE current density over applied field (J–E) is theoretically explained by the modified Fowler–Nordheim (F–N) equation,21where, J is the emission current density, E is the applied electric field, a and b are constants, typically 1.54 × 10−6 A eV V−2 and 6.83 eV−3/2 V nm−1, respectively. ϕ is the workfunction of the emitter material, λM is the macroscopic pre-exponential correction factor, νF is value of the principal Schottky–Nordheim barrier function (a correction factor), and β is the field enhancement factor. The observed J–E characteristic further is analyzed by plotting a graph of ln(J/E2) versus, (1/E) known as the Fowler–Nordheim (F–N) plot. The Fig. 5(b) shows the corresponding F–N plots, which exhibit overall nonlinear behaviour over the entire range of the applied field, indicating semiconducting nature of the emitters. Similar nonlinear F–N plots have been reported for other semiconductors.14–20,22
| Sr. no. | Morphology | Turn-on fielda (V μm−1) | Threshold field (V μm−1) | Reference |
|---|---|---|---|---|
| a The turn-on field, defined as field required draw an emission current density of 10 μA cm−2. | ||||
| 1 | Bi2S3 nanoflowers | — | 2.2 (90 μA cm−2) | 14 |
| 2 | Bi2S3 nanoflowers | 7.45 | — | 15 |
| 3 | Bi2S3 nanowires | — | 3.52 (63 μA cm−2) | 16 |
| 4 | In2Se3 nanowires | 4.17 | — | 17 |
| 5 | Cu2O–ZnO nanobrush | 6.5 | 10.5 (∼425 μA cm−2) | 18 |
| 6 | Cu2S–ZnO nanoflowers | 2 | — | 19 |
| 7 | CeO2–ZnO heteroarchitectures | 1.8 | 3.2 (∼364 μA cm−2) | 20 |
| 8 | CdS–Bi2S3 heteroarchitectures | 1.8 | 3.1 (∼677 μA cm−2) | Present study |
The mechanism of field enhancement is suggested to be essentially different in this case because of hierarchical architectures. The superior emission behaviour exhibited by the CdS–Bi2S3 heteroarchitecture emitter can be attributed to combined effect due to:
(I) Unique morphological pattern of an emitter: in such hierarchical emitter, the epitaxial grown CdS nanorods (branches) on the Bi2S3 nanorods (stem) play a vital role in 'local' field enhancement. According to ‘two-stage’ approach, the ‘first’ enhancement in the local field occurs due to ‘stem’. This field is further enhanced at the ‘branches’ owing to their high aspect ratio.23
(II) Increase in the number of emission sites: the growth of CdS nanorods over the Bi2S3 nanorods remarkably increase the number of ‘potential’ emission sites in comparison with the pristine Bi2S3 nanorods, as the strength of ‘local’ electric field at the apex of the CdS nanostructures will be higher than that of Bi2S3 nanorods.
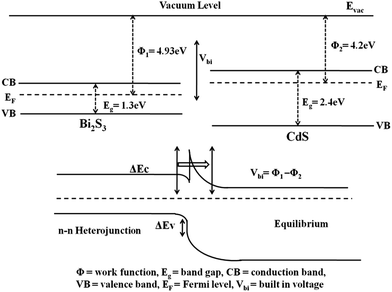
(III) Modulation of work function: the electronic properties of the hierarchical emitter are modulated by the ‘interface’ between the two nanostructures. A schematic of the energy band diagram obtained using Anderson rule for n–n type semiconductor junction is depicted below. Both the CdS and Bi2S3 are n-type semiconductors possessing charge carrier concentration (at room temperature) ∼1.19 × 1019 and 3 × 1018 cm−3, respectively.24,25 It indicates that the conduction band minimum of CdS will lie slightly below that of Bi2S3, when the Fermi levels are aligned. Under the application of applied field, at the interface charge transfer (electron transport via tunnelling) is expected to take place from conduction band of Bi2S3 to conduction band of CdS. Thus the enhanced charge carrier density in conduction band of CdS rationally contributes to the emission current density drawn from the heteroarchitecture emitter (Scheme 1).
Along with the emission characteristics, the field emission current stability is one of the important parameters in the context of practical applications as cold cathode. The emission current versus time (I–t) plots corresponding to the preset values of 1 and 5 μA, recorded at a base pressure of 1 × 10−8 mbar with a sampling time of 10 s for more than 3 hours duration are shown in Fig. 5(c) and (d). The CdS–Bi2S3 heteroarchitecture emitter exhibits better emission stability as compared to the pristine Bi2S3, and the current fluctuations are observed to be within ±10% of the average value. The current fluctuations may be due to adsorption, desorption of the residual gas atoms/molecules on the emitter surface. The field emission images are shown in inset of the Fig. 5(c) and (d). The image shows a number of tiny bright spots, corresponding to the emission from the most protruding nanostructures on the emitter surface. We have also studied the post field emission surface morphology of the emitters, and interestingly the SEM images (not depicted here for sake of brevity) show no noticeable deterioration of the emitter surface indicative of robust nature of the CdS–Bi2S3 heteroarchitecture.
Photodetection
The as synthesized Bi2S3 nanoflowers and CdS–Bi2S3 heteroarchitecture were also tested for different LED and broadband light photodetection. Fig. 6(a) shows I–V characteristics of Bi2S3 nanoflowers based photosensor under light illumination for different LED's at applied voltage of 5 V. It is clear from the plots that resistance of the material decreases with the illumination of light. Fig. 6(c) and (e) shows the typical I–t plot for the Bi2S3 nanoflowers based sensor upon illumination of blue and red LEDs for applied bias of 2 V. The response time of the sensor can be defined as the time taken to reach 90% of the maximum current value observed under illumination. Similarly, the recovery time is defined as the time taken by the sensor when the photocurrent value drops to 10% of its maximum value.26 The response and recovery times for the Bi2S3 nanoflowers based sensor under the illumination of blue and red LEDs are given in the Table 2. The Fig. 6(b) shows I–V characteristics of the CdS–Bi2S3 heteroarchitecture based photosensor studied under the illumination of different LED's at an applied voltage of 5 V. The plot 6(b) shows increment in the photocurrent due to the decoration of CdS nanostructures over the Bi2S3 nanoflowers. The Fig. 6(d) and (f) shows the typical I–t plot for the CdS–Bi2S3 heteroarchitecture based photosensor upon illumination of blue and red LED, respectively. Table 2 represents comparison of the response and recovery times of both the photosensor under illumination of blue, red LED's at an applied bias of 2 V. The I–t plots for green, yellow and orange LED are given in S4.| LED types | Bi2S3 photosensor | CdS–Bi2S3 photosensor | ||
|---|---|---|---|---|
| Response time (sec) | Recovery time (sec) | Response time (sec) | Recovery time (sec) | |
| Blue | 1.6 | 4.5 | 4.42 | 4.8 |
| Red | 1.6 | 4.53 | 4.1 | 4.6 |
| White light | 655 (ms) | 678 (ms) | 207 (ms) | 315 (ms) |
The typical I–V plots corresponding to white light illumination of the Bi2S3 and CdS–Bi2S3 photosensors, recorded at an applied voltage of 5 V, are depicted in Fig. 7(a) and (b), respectively. It is clearly seen from the I–V plots that white light illumination leads to generation of large photocurrent, in both the photosensors, as compared to the LEDs illumination. The typical I–t plots for the Bi2S3 and CdS–Bi2S3 photosensors measured at bias voltage of 0.5 V are shown in Fig. 7(c) and (d). Fig. 7(e) and (f) represent the I–t behaviour of both the photosensors corresponding to a single photo-switching cycle. The response and recovery times for both of the sensors for white light detection, calculated from the Fig. 7(e) and (f), are observed to be lower than that of different LEDs. Furthermore, the CdS–Bi2S3 heteroarchitecture photosensor exhibits lower values of response and recovery time as compared to Bi2S3 photosensor (Table 2).
The light detection mechanism of the photosensors can be explained using photoconductivity effect.27 when light incident on the surface of Bi2S3 nanoflowers then electrons present in the material forms excitons which then are detached and accelerated by an external applied voltage leads to generation of photocurrent. In order to generate photocurrent the required condition is the energy of the incident photon should be greater than the band gap of the material. In case of CdS–Bi2S3 heteroarchitecture we observed large photocurrent as compared to Bi2S3 nanoflowers because of lowering in bandgap value. There are many reports on the photodetector based on 2D layered materials such as MoS2,28,29 WS2,30 MoSe2,31 WSe2,32,33 SnS2,34 black phosphorous35 etc. exhibits superior photocurrent generation with good response and recovery time. Our results also show better response and recovery time like 2D layered materials.
Conclusion
In conclusion, we have successfully synthesized Bi2S3 nanoflowers and CdS–Bi2S3 heteroarchitecture by using single step hydrothermal method. We also report here Bi2S3 nanoflowers and CdS–Bi2S3 heteroarchitecture based field emitters and photodetector. In the field emission investigation the turn-on value to extract current density of 10 μA cm−2 was found to be ∼2.9 V μm−1 and ∼1.8 V μm−1 for Bi2S3 nanoflowers and CdS–Bi2S3 heteroarchitecture, respectively. The broadband photodetection study reveals that as synthesized materials are highly responsive and possesses better response and recovery time under the illumination of visible light. The Bi2S3 nanoflowers based photodetector shown response and recovery time of ~655 ms and 678 ms respectively. Similarly, CdS–Bi2S3 heteroarchitecture based photodetector possesses response and recovery time of ~207 ms and 315 ms respectively. Our results open up new windows for the as synthesized materials in various nanoelectronic and optoelectronic devices such as FET, humidity sensor, gas sensor etc.Conflict of interest
The author declares no competing financial interest.Acknowledgements
P. K. Bankar acknowledges SPPU and DST for the financial support. Prof. M. A. More would like to thank the BCUD, of Savitribai Phule Pune University for the financial support provided for the field emission work under CNQS-UPE-UGC program activity. S. S. Warule gratefully acknowledges the financial support from BCUD, SPPU, Pune. The research work was supported by Department of Science and Technology (Government of India) under Ramanujan Fellowship to Dr D. J. Late (Grant No. SR/S2/RJN-130/2012), NCL-MLP project grant 028626, DST-SERB Fast-track Young scientist project Grant No. SB/FT/CS-116/2013, Broad of Research in Nuclear Sciences Grant No. 34/14/20/2015 (Government of India) and the partial support by INUP IITB project sponsored by DeitY, MCIT, Government of India. Authors would like to thank Director, C-MET, Pune for Characterization of samples.Notes and references
- G. Sheet, U. K. Gautam, A. D. Thakur, K. Hirata, Y. Bando and T. Nakayama, Appl. Phys. Lett., 2009, 94, 053108 CrossRef
.
- Z. Fang, Y. Liu, Y. Fan, Y. Ni, X. Wei, K. Tang and Y. Chen, J. Phys. Chem. C, 2011, 115, 13968–13976 CAS
.
- H. Kim, S. Jeon, M. Lee, J. Lee and K. Yong, J. Mater. Chem., 2011, 21, 13458–13463 RSC
.
- S. S. Warule, N. S. Chaudhari, R. T. Khare, J. D. Ambekar, B. B. Kale and M. A. More, CrystEngComm, 2013, 15, 7475–7483 RSC
.
- W. Zhang, C.-P. Chuu, J.-K. Huang, C.-H. Chen, M.-L. Tsai, Y.-H. Chang, C.-T. Liang, Y.-Z. Chen, Y.-L. Chueh, J.-H. He, M.-Y. Chou and L.-J. Li, Sci. Rep., 2014, 4, 3826 Search PubMed
.
- H. Qiao, J. Yuan, Z. Xu, C. Chen, S. Lin, Y. Wang, J. Song, Y. Liu, Q. Khan, H. Y. Hoh, C.-X. Pan, S. Li and Q. Bao, ACS Nano, 2015, 9, 1886–1894 CrossRef CAS PubMed
.
- H. Zhang, X. Zhang, C. Liu, S. T. Lee and J. Jie, ACS Nano, 2016, 10, 5113–5122 CrossRef CAS PubMed
.
- R. P. Panmand, Y. A. Sethi, R. S. Deokar, D. J. Late, H. M. Gholap, J. O. Baeg and B. B. Kale, RSC Adv., 2016, 6, 23508–23517 RSC
.
- K. Yao, Z. Y. Zhang, X. L. Liang, Q. Chen, L. M. Peng and Y. Yu, J. Phys. Chem. B, 2006, 110, 21408–21411 CrossRef CAS PubMed
.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed
.
- N. S. Ramgir, D. J. Late, A. B. Bhise, I. S. Mulla, M. A. More, D. S. Joag and V. K. Pillai, Nanotechnology, 2006, 17, 2730 CrossRef CAS
.
- M. S. Pawar, P. K. Bankar, M. A. More and D. J. Late, RSC Adv., 2015, 5, 88796–88804 RSC
.
- R. H. Fowler and L. Nordheim, Proc. R. Soc. London, Ser. A, 1928, 119, 173–181 CrossRef CAS
.
- S. S. Warule, R. V. Kashid, D. R. Shinde, N. S. Chaudhari, B. B. Kale and M. A. More, Architectured Bi2S3 nanoflowers: photoenhanced field emission study, J. Nanopart. Res., 2012, 14(6), 1–13 CrossRef
.
- X. Yu and C. Cao, Cryst. Growth Des., 2008, 8, 3951–3955 CAS
.
- S. S. Warule, N. S. Chaudhari, B. B. Kale, S. Pandiraj, R. T. Khare and M. A. More, Cryst. Growth Des., 2013, 15, 890–896 CAS
.
- S. R. Suryawanshi, P. K. Bankar, M. A. More and D. J. Late, RSC Adv., 2015, 5, 65274–65282 RSC
.
- M. Deo, D. Shinde, A. Yengantiwar, J. Jog, B. Hannoyer, X. Sauvage and S. Ogale, J. Mater. Chem., 2012, 22, 17055–17062 RSC
.
- S. Li, K. Yu, Y. Wang, Z. Zhang, C. Song, H. Yin and Z. Zhu, Cryst. Growth Des., 2013, 15, 1753–1761 CAS
.
- S. S. Warule, N. S. Chaudhari, B. B. Kale, K. R. Patil, P. M. Koinkar, M. A. More and R. I. Murakami, J. Mater. Chem., 2012, 22, 8887–8895 RSC
.
- R. G. Forbes, Elev. Int. Vac. Microelectron. Conf. IVMC'98, Cat. No. 98TH8382, 1998, p. 534 Search PubMed
.
- A. A. Tabbakh, M. A. More, D. S. Joag, I. S. Mulla and V. K. Pillai, ACS Nano, 2010, 4, 5585–5590 CrossRef PubMed
.
- V. V. Zhirnov, E. I. Givargizov and P. S. Plekhanov, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 1995, 13(2), 418–421 CrossRef CAS
.
- S. Kose, F. Atay, V. Bilgin, I. Akyuz and E. Ketenci, Appl. Surf. Sci., 2010, 256(13), 4299–4303 CrossRef CAS
.
- O. Madelung, Semiconductors Data Handbook, Springer, Berlin, 2004, p. 691 Search PubMed
.
- M. B. Erande, M. S. Pawar and D. J. Late, ACS Appl. Mater. Interfaces, 2016, 8, 11548–11556 CAS
.
- M. M. Furchi, D. K. Polyushkin, A. Pospischil and T. Mueller, Nano Lett., 2014, 14, 6165–6170 CrossRef CAS PubMed
.
- W. Zhang, C. P. Chuu, J. K. Huang, C. H. Chen, M. L. Tsai, Y. H. Chang, C. T. Liang, Y. Z. Chen, Y. L. Chueh, J. H. He, M. Y. Chou and L. J. Li, Sci. Rep., 2014, 4, 3826 Search PubMed
.
- C. Chen, H. Qiao, S. Lin, C. M. Luk, Y. Liu, Z. Xu, J. Song, Y. Xue, D. Li, J. Yuan, W. Yu, C. Pan, S. Lau and Q. Bao, Sci. Rep., 2015, 5, 11830 CrossRef PubMed
.
- C. Zhang, S. Wang, L. Yang, Y. Liu, T. Xu, Z. Ning, A. Zak, Z. Zhang, R. Tenne and Q. Chen, Appl. Phys. Lett., 2012, 100, 243101–243105 CrossRef
.
- J. Xia, X. Huang, L. Z. Liu, M. Wang, L. Wang, B. Huang, D. D. Zhu, J. J. Li, C. Z. Gu and X. M. Meng, Nanoscale, 2014, 6, 8949–8955 RSC
.
- W. Zhang, M. H. Chiu, C. H. Chen, W. Chen, L. J. Li and A. T. S. Wee, ACS Nano, 2014, 8, 8653–8661 CrossRef CAS PubMed
.
- Z. Zheng, T. Zhang, J. Yao, Y. Zhang, J. Xu and G. Yang, Nanotechnology, 2016, 27, 225501 CrossRef PubMed
.
- G. Su, V. G. Hadjiev, P. E. Loya, J. Zhang, S. Lei, S. Maharjan and H. Peng, Nano Lett., 2014, 15, 506–513 CrossRef PubMed
.
- M. Buscema, D. J. Groenendijk, S. I. Blanter, G. A. Steele, H. S. J. Zant and A. C. Gomez, Nano Lett., 2014, 14, 3347–3352 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21085f |
| ‡ Prashant K. Bankar and Mahendra S. Pawar contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |