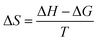Interaction between mercuric chloride and extracellular polymers of biofilm-forming mercury resistant marine bacterium Bacillus thuringiensis PW-05
Hirak R. Dash and
Surajit Das*
Laboratory of Environmental Microbiology and Ecology (LEnME), Department of Life Science, National Institute of Technology, Rourkela-769 008, Odisha, India. E-mail: surajit@nitrkl.ac.in; surajit@myself.com; Fax: +91 661 2462022; Tel: +91 661 2462684
First published on 11th November 2016
Abstract
The interaction mechanism of mercury (Hg2+) with extracellular polymers (EPS) produced from a mercury resistant marine bacterium Bacillus thuringiensis PW-05 was studied. Extracted EPS contained 1546.93 ± 53.63 μg ml−1 of carbohydrate, 156.70 ± 6.77 μg ml−1 of protein and 3.78 ± 0.29 μg ml−1 of DNA. The zeta potential was increased from −9.08 ± 0.12 mV of pristine EPS to −1.39 ± 0.07 mV of mercury interacted EPS. >90% of Hg2+ was absorbed by the EPS from the solution after 2 h. UV-Vis, FTIR and 1H NMR spectroscopy revealed the possible role of carboxyl, phosphoryl, hydroxyl, amino and sulfhydryl functional groups in the interaction process. EPS architecture was found to be modified from purely amorphous to fairly crystalline after interaction with mercury as revealed by XRD. The interaction process was found to be spontaneous with negative free energy (−61.77, −63.17 and −61.74 J mol−1 at 298, 308 and 318 K respectively) and an enthalpy driven process.
1. Introduction
Mercury is ubiquitous in the environment as a natural element. Degassing from the earth's crust constitutes one of the most copious natural sources of mercury (Hg) pollution in the environment that accounts for approximately 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year. However, anthropogenic sources contribute about 20
000 tonnes per year. However, anthropogenic sources contribute about 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of Hg each year.1 Hg pollution is considered to be a huge threat to the universe as it is the most toxic among metals and possesses a high residence time of more than two years.2 Though a biogeochemical cycle of Hg exists in the natural ecosystem, it has the potential to cause mischief to the natural flora and fauna at each trophic level. On entering the food chain, it is accumulated and its level gradually increases at each trophic level so that human beings, who are at the top of the food chain, become the worst sufferers.3 Methylation of inorganic Hg (Hg2+), mediated by certain sediment bound sulfate reducing bacteria worsens the situation by converting Hg2+ to its most toxic form i.e. organic mercury.4 In this regard, Hg removal from the environment should be of high importance and warrants advanced research.
000 tonnes of Hg each year.1 Hg pollution is considered to be a huge threat to the universe as it is the most toxic among metals and possesses a high residence time of more than two years.2 Though a biogeochemical cycle of Hg exists in the natural ecosystem, it has the potential to cause mischief to the natural flora and fauna at each trophic level. On entering the food chain, it is accumulated and its level gradually increases at each trophic level so that human beings, who are at the top of the food chain, become the worst sufferers.3 Methylation of inorganic Hg (Hg2+), mediated by certain sediment bound sulfate reducing bacteria worsens the situation by converting Hg2+ to its most toxic form i.e. organic mercury.4 In this regard, Hg removal from the environment should be of high importance and warrants advanced research.
Extracellular polymers/polymeric substances (EPS) are microbial products whose major constituents are polysaccharides and proteins.5 EPS secretion mediates the initial attachment of cells to any substrata to form the structure and architecture of the microbial biofilm.6 EPS matrix attributes various properties to microbial biofilm such as attachment, detachment, tensile strength, resistance to antibiotics and metals and exo-enzymatic degradation of detrimental substances.7 As EPS are comprised of high molecular weight compounds with charged functional groups, it possesses both adsorptive and adhesive properties. The charged moieties serve as a natural ligand source by providing binding sites for other charged molecules including metals.8 Studies by Langmuir's model predicted the same binding site on EPS for the metal ions such as cadmium and zinc with a preferential binding of lead.9 A detailed mechanistic study on the interaction mechanism between bacterial EPS and uranium elucidated the involvement of different binding sites such as, binding of organic phosphate and carboxyl groups, and chelation by phosphate bodies in a marine bacterium Idiomarina loihiensis MAH1.10 Thus, microorganisms-produced macromolecules collectively called EPS have received attention as novel biosorbents.11
From environmental perspective, biosorption is the uptake of contaminants by any biological material by different physico-chemical processes such as ion exchange, sorption, complexation, chelation and micro-precipitation. Though bioavailability of metals is mostly dependant on many environmental factors such as pH, alkalinity, redox potential and de novo potential of the microorganisms; there are numerous reports available on biosorption of metals by bacterial EPS.12–16 Liu et al.17 reported a high level of removal of Zn2+, Cu2+, Cr3+, Cd2+, Co2+ and Ni2+ by EPS from activated sludge of municipal waste water. EPS produced by diverse group of bacteria such as Gram-positive Bacillus,18 Gram-negative Pseudomonas19,20 and Cyanobacteria Synechoeystis sp.21 have also been reported to remove significant amounts of metals from the environment.
Marine environment is considered to be a poly-extreme habitat. Thus, the inhabitant microorganisms adapt in various ways to overcome the noxious conditions. Secretion of metabolites and EPS is one of the major modes of their adaptation.22 EPS production in marine bacteria costs a lot, consuming up to 70% of the total energy and significant carbon investment; however, the benefits conveyed are significantly higher in terms of the enhanced growth and survival in extreme environments.23 In the marine environment, EPS have many implications, such as removal of metals, transfer of nutrients to marine microorganisms and mineral sorption.24 EPS also play an important role in supplementing carbon demand of other marine fauna, which feed on microbial aggregates, microbial mats and biofilms.8,25 Many reports have confirmed higher level of EPS production from marine bacteria that increases their adaptability to the extreme environmental conditions.26–29 As a metabolic end product of bacteria, EPS are accumulated on their surface, providing a good binding site for nutrients and metal ions for their adsorption and transportation.30 In general, the binding of metals by microbial adsorption occurs on the cell surface. Microbial derived metabolites, polysaccharides and other cell wall constituents have been reported to be capable of uptake and accumulation of metal ions.31 Thus, EPS enable protection to the bacterial community and facilitate access of nutrients to the bacterial cells.32 The acidic property of the EPS is conferred by the presence of phosphate and carboxylate groups which possess a huge potential to bind with the positively charged metal ions present in the contaminated environment.33 However, little information is available till date regarding the mechanism of interaction between EPS and toxic metals such as Hg. The current work aims to obtain an illustrated mechanism of interaction between EPS and Hg2+.
2. Experimental
Strain selection, characterization and growth medium
Bacillus thuringiensis PW-05, previously isolated and characterized from the marine environment of Bay of Bengal, India, was used in this study. The isolate has been characterized for its tolerance to mercury and antibiotics, biofilm formation, resistance mechanism to Hg and its removal potential.29 Due to its marine origin and mercury resistance nature, sea water nutrient (SWN) agar (peptone – 5.0 g, yeast extract – 3.0 g, agar – 15.0 g, aged sea water – 500 ml, deionized water – 500 ml, pH – 7.5 ± 0.1) medium supplemented with 10 ppm of Hg as HgCl2 was used as the routine growth medium. The isolate was identified by 16S rRNA gene sequencing and the sequence has been submitted to NCBI database to obtain the accession number JX273776.EPS extraction and analysis of EPS
Mass culture of B. thuringiensis PW-05 was done in a bioreactor (New Brunswick, BioFlo/CelliGen 115) in Davis minimal broth (dipotassium phosphate – 7.0 g, monopotassium phosphate – 2.0 g, sodium citrate – 0.5 g, magnesium sulphate – 0.1 g, ammonium sulphate – 1.0 g, distilled water – 1000 ml, pH 7.0 ± 0.2) supplemented with 1% glucose solution and 20 mM CaCl2 solution. Over-night grown 0.5 McFarland culture was inoculated in the culture medium at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The isolate was grown under controlled environmental conditions (temperature – 37 °C, pH – 7.15, DO – 50%, agitation – 50 rpm) for 48 h and the mass culture was used for further extraction of EPS. EPS extraction was carried out using a slightly modified protocol of Smitinont et al.34 following Chakraborty and Das.35 Mass culture of bacterium from Davis minimal broth was centrifuged at 6000 rpm for 30 min at 4 °C to remove the bacterial cells. The supernatant was precipitated with 2.2 volume of chilled ethanol (50%) by incubating the mixture at 4 °C for 24 h. Precipitated EPS was collected by centrifugation at 6000 rpm for 30 min at 4 °C. The pellet containing EPS was stored at room temperature and the supernatant was precipitated repeatedly by 70% and absolute ethanol following the above protocol. Finally, the collected pellets containing EPS were air dried, lyophilized and stored at room temperature till further use.
100. The isolate was grown under controlled environmental conditions (temperature – 37 °C, pH – 7.15, DO – 50%, agitation – 50 rpm) for 48 h and the mass culture was used for further extraction of EPS. EPS extraction was carried out using a slightly modified protocol of Smitinont et al.34 following Chakraborty and Das.35 Mass culture of bacterium from Davis minimal broth was centrifuged at 6000 rpm for 30 min at 4 °C to remove the bacterial cells. The supernatant was precipitated with 2.2 volume of chilled ethanol (50%) by incubating the mixture at 4 °C for 24 h. Precipitated EPS was collected by centrifugation at 6000 rpm for 30 min at 4 °C. The pellet containing EPS was stored at room temperature and the supernatant was precipitated repeatedly by 70% and absolute ethanol following the above protocol. Finally, the collected pellets containing EPS were air dried, lyophilized and stored at room temperature till further use.
The extracted EPS was treated with 10 ppm of mercury as HgCl2 and the mixture was kept in a rotary shaker for 24 h at 37 °C. Protein and carbohydrate content in the pristine EPS as well as the EPS interacted with Hg2+ was measured by Lowry method and anthrone method using bovine serum albumin and glucose respectively as standards.36,37 The experiments were performed in triplicates and the F-value (two way ANOVA) were calculated and compared with tabulated values to determine the significant variation among the EPS constituents of pristine EPS and Hg2+ interacted EPS. Additionally, the surface charge of the EPS was measured using zeta potential.38 DNA content of the extracted EPS was determined by quantifying the double stranded (DS) as well as single stranded (SS) DNA using BioPhotometer® D30 (Eppendorf, Germany) using the principle of micro volume sample retention system.39 Induction of DNA damage by mercury was assessed using calf thymus DNA (Sigma-Aldrich, USA) as the reference DNA. Different concentration of mercury (10, 25, 50 ppm) were treated with constant amount of DS calf thymus DNA (50 μg ml−1) followed by the quantification of DS and SS DNA at an interval of 30 min, 60 min, 90 min, 120 min and 24 h at 260 nm absorbance. The experiment was conducted in triplicates with suitable negative control.
Hg2+ absorption capacity of EPS
100 mg l−1 of EPS was mixed with 10, 25 and 50 ppm of HgCl2 (upto the maximum tolerance level of the source isolate). The solutions were put in an oscillator at 37 °C and the residual mercury in the solution was estimated at 30, 60, 90, 120 min and 24 h by atomic absorption spectrophotometer (AAS) (AANALYST-400, Perkin-Elmer, USA). A negative control of EPS without any mercury supplement was included in this study.Spectroscopic analyses
Prior to analysis, pristine EPS and HgCl2 were dissolved in sterile phosphate buffer solution (0.2 mol l−1, pH 7.4). A total sample volume of 25 ml was prepared from different volumes of EPS and HgCl2 solution. The final concentration of EPS in the sample was maintained at 100 mg l−1 while the concentration of HgCl2 varied from 0, 10, 25, 50 and 100 ppm. The mixture was incubated for 24 h at room temperature before further analysis. The resultant solution was mixed properly by vortex. The samples were lyophilized and characterized by various spectroscopic techniques such as UV-Vis spectrophotometry, FTIR, XRD and 1H NMR spectroscopy. UV-Vis spectroscopy was conducted with all the samples, however, the FTIR, XRD and 1H NMR were carried out with the EPS treated with 50 ppm of mercury as HgCl2, the concentration similar to the tolerance level of the isolate.UV-Visible absorption spectra were measured on a Lambda 35 spectrophotometer (Perkin-Elmer, Germany) with scanning wavelength from 200 to 700 nm at 0.1 nm increments.
FTIR spectra were recorded in a Perkin-Elmer RX I FTIR spectrophotometer. The transmittance of each sample was analyzed in the wavelength ranged from 4000 to 400 cm−1 with an average of 64 scans. The samples in the form of pellets were prepared by pressing mixtures of 100 mg of chromatographic grade KBr with 1 mg dry powdered lyophilized samples under vacuum conditions to avoid moisture uptake.
1H NMR spectroscopy was carried out in a Bruker 400 MHz spectrometer at room temperature. The instrument was equipped with a 5 mm inverse probe with z-gradient coil. The chemical shifts with response to the interaction between EPS and HgCl2 has been reported in δ with respect to tetramethylsilane (TMS). All NMR experiments have been carried out in D2O (Sigma-Aldrich, USA).
X-ray diffraction (XRD) profile of the prepared samples was collected on a X-ray diffractometer (Rigaku Miniflex, Japan) using nickel filtered Cu Kα radiation (k = 1.54056 Å) and scanned from 10° to 60° at room temperature with a scan rate of 3° min−1.
Thermodynamics of EPS–Hg interaction
The binding studies of EPS with Hg have been carried out by UV-Vis spectroscopic technique. The stock of solutions of Hg2+ and EPS were prepared in phosphate buffer solution (pH 7.4). To a particular amount of EPS (100 mg l−1), definite amounts of Hg2+ were added so as to obtain a final volume of 20 ml. The concentrations of Hg2+ varied upto 100 ppm. The solutions were put in an oscillator and left to attain equilibrium for 24 h at room temperature and then analysed by UV-Vis spectral studies. In this study, Langmuir adsorption isotherm has been employed to describe the interaction between Hg2+ and EPS.40 The equilibrium concentration was determined by measuring the absorbance spectrophotometrically at λmax = 305 nm. The amount of bound Hg onto EPS was calculated according to the following equation:41
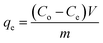 | (1) |
The adsorption isotherms were also obtained from the above studies. The effect of temperature on the equilibrium concentration of the adsorbed Hg2+ ions was studied to determine the corresponding thermodynamic parameters. For this purpose, the solutions were shaken in an isothermal shaker maintained at 298, 308 and 318 K.
3. Results and discussion
Characterization of Bacillus thuringiensis PW-05
The bacterium B. thuringiensis PW-05 has been reported to tolerate high levels of toxic metals such as mercury (50 ppm), cadmium (160.8 ppm), zinc (807.35 ppm), lead (1250 ppm) and arsenic (232.4 ppm), in addition to being resistant to various antibiotics such as amoxicillin, cephradine, ampicillin and methicillin. The tolerance level to mercury by the isolate B. thuringiensis PW-05 was higher than the tolerance level of other reported mercury resistant bacteria i.e. Bacillus cereus (8.14 ppm), Lysinibacillus sp. (16.29 ppm), Bacillus sp. (27.15 ppm), Microbacterium oxydans (27.15 ppm), Serratia marcescens (16.29 ppm), Ochrobactrum sp. (16.29 ppm),42 Vibrio sp. (0.08 ppm)28 and Oceanobacillus iheyensis (27.15 ppm).43 The isolate harbours the mercury resistant mer operon in its plasmid and is capable of removing up to 94.72% of inorganic mercury in vitro. Many studies have revealed the chance of loss of plasmid due to repeated sub-culturing, long term storage, and growth in absence of stress conditions.44–46 Thus, in order to protect the plasmid harboring mer operon in B. thuringiensis PW-05, the growth medium was supplemented with mercury as HgCl2. The isolate was also capable of forming biofilm both in presence (50 ppm of mercury as HgCl2) and absence of mercury with a relatively higher amount of biofilm formation in presence of mercury (data not shown here).Extracellular polymeric substances (EPS) is a term used to describe the aggregation of organic macromolecules such as polysaccharides, proteins, nucleic acids, lipids and other polymeric compounds found both on the outer surface of cell wall and in the interior of microbial assemblages.47 EPS secreted by bacteria are responsible for cell aggregation, cell adhesion and biofilm formation to overcome hostile environmental conditions.48 Hence, biofilm forming nature of the bacterial isolate increases the efficacy of EPS production which can be utilized further for interaction and subsequent removal of metal ions from the environment.6,49 In contrary, it may cause problems in the food industry, since it is responsible for persistent human infections by increased antibiotic resistance, material deterioration and economic loss.27 From environmental bioremediation point of view, EPS play pivotal role due to their involvement in flocculation and binding of metal ions from solutions. Use of this biopolymer for biosorption of toxic substances is more effective and safer alternative to other chemical techniques available now-a-days i.e. precipitation, coagulation, ion-exchange, electrochemical and membrane process. The major advantage of using these non-living bacterial secretions is their easy availability and non-pathogenic nature.11,49,50
In the marine environment, inhabitant microorganisms are exposed to the noxious, dynamic conditions of pH, salinity and sea surface temperature22 and to overcome those circumstances, secretion of EPS acts as a mode of adaptation.50 Most of the marine bacteria possess a greater ability to synthesise EPS and the marine bacterium used in this study, B. thuringiensis PW-05, produced high amount of EPS. Many potential EPS producing, biofilm forming bacteria have also been reported from marine environments such as Vibrio parahaemolyticus,51 Oceanobacillus iheyensis,52 archaea,53 Bacillus subtilis,54 and Hymenobacter aerophilus.33
Extraction of EPS and its characterization
The major constituents of the extracted EPS were found to be proteins, carbohydrates and nucleic acids as obtained in the present study. These are the most common constituents of EPS as reported by Tsuneda et al.55 and Jain et al.56 A total of 182.7 mg of EPS was extracted from 800 ml of bacterial culture after lyophilisation with optimum growth conditions in the bioreactor. During the isolation of bacterial EPS, the procedure should consider the following points i.e. optimal recovery of EPS with no or minimal inclusion of non-EPS components and avoidance of harsh chemicals to prevent modification of EPS structure. In this regard, precipitations with different alcohols represent a common detection, isolation, and purification method for EPS.57 Several studies involving bacterial EPS followed the chemical method of EPS extraction by using chilled ethanol precipitation technique.34,35,53,56,58,59 Additionally, extraction of bacterial EPS with a series of ethanol extraction steps using different concentrations of ethanol (i.e. 50%, 70% and 100% as used in this study) has also been reported to yield approximately 98% (w/w) purity.60The carbohydrate and protein content of the extracted EPS in the present study was determined as 1546.93 ± 53.63 μg ml−1 and 150.70 ± 6.7 μg ml−1 respectively. Additionally, 3.78 ± 0.29 μg ml−1 of DS DNA was determined in the EPS sample (Table 1). The zeta potential of the pristine EPS sample was found to be −9.087 ± 0.12 mV with corresponding conductivity of 1.06 mS cm−1; whereas, the zeta potential of HgCl2 interacted EPS was −1.39 ± 0.07 mV with the conductivity of 81.8 mS cm−1. However, none of the parameters showed statistically significant variation after interaction with Hg2+ (ANOVA, P > 0.05). Bacterial cells exhibit a total negative charge (−20.7 mV as observed in this study for B. thuringiensis PW-05 cells) due to the presence of cell surface lipopolysaccharides, teichoic acids and EPS.61 Though statistically insignificant, the increase in negativity of zeta potential of EPS upon interaction with Hg2+ may be due to the binding efficacy of Hg2+ with EPS. Additionally, the increase in negativity of the zeta potential between the whole bacterial cell (−20.7 mV) and pristine EPS (−9.087 mV) might be due to the absence of cell components contributing to the negative charge of a cell in the extracted EPS. In a similar line, many studies have also revealed an increase in EPS resulting in less negative zeta potential values.55,62 This clearly indicates the binding efficacy of Hg2+ with EPS.
| Constituents/characteristics | Concentration/amount (mean ± SD) | |
|---|---|---|
| Pristine EPS | EPS with Hg | |
| Carbohydrate | 1546.93 ± 53.63 μg ml−1 | 1510 ± 65.87 μg ml−1 |
| Protein | 156.70 ± 6.77 μg ml−1 | 152.60 ± 7.40 μg ml−1 |
| DNA | 3.78 ± 0.29 μg ml−1 | 0.56 ± 0.08 μg ml−1 |
| Zeta potential | −9.08 ± 0.12 mV | −1.39 ± 0.07 mV |
The decrease in the quantity of DS DNA from 3.78 ± 0.29 μg ml−1 to 0.56 ± 0.08 μg ml−1 might be due to the strand break in DNA induced by mercury.63 Time scale experiment involving the co-incubation of calf-thymus DS DNA (50 μg ml−1) with different concentrations of mercury (10, 25 and 50 ppm) indicates that the amount of DS DNA is decreased with an increase in time (from 30 min to 24 h). Thus, the decrease in the amount of the DS DNA is attributed to the interaction of mercury with the DS DNA and formation of SS DNA. The ratio between SS to DS DNA was found to vary between 0.67 to 1.25 (with 10 ppm of HgCl2), 0.76 to 1.26 (with 25 ppm of HgCl2) 0.85 to 1.41 (with 50 ppm of HgCl2) with the increase of time. The decrease of DS DNA samples in presence of mercury has also been well established.64 Shamsi and Kraatz65 predicted the canonical Watson–Crick base pairs as well as the non-canonical base pairs to be the sites of interaction between nucleic acids and metals. Thus, the decrease in the amount of DS DNA in bacterial EPS upon interaction with mercury might be due to the strand breaks induced by mercury.66
Hg2+ absorption capacity of EPS
An increasing trend of Hg absorption was obtained irrespective of the initial concentration of mercury supplement till 24 h (Fig. 1). More than 90% mercury was found to interact with EPS after 2 h at 37 °C. Thereafter, increase in exposure time upto 24 h resulted in slight increase of Hg absorption (91.3% to 92.84% for 10 ppm Hg, 92.5% to 93.61% for 25 ppm and 93.85% to 95.6% for 50 ppm of initial Hg supplement). This suggests the saturation of EPS for binding of Hg within 2–24 h. However, 100% absorption of Hg was not obtained and in line with the obtained results none of the previous experiments have reported to achieve 100% absorption capacity of EPS with mercury or other metals tested.67–69 Maximum adsorption capacity of different heavy metals also followed the same trend as reported in some earlier studies.40,70 Several studies have demonstrated the metal sorption capacity of extracellular biopolymers that has been extracted from pure bacterial cultures as portrayed in the present study.67,68 The absorption capability of Hg2+ follows the same trend of absorption capabilities of EPS for other heavy metals, e.g. 83.8%, 92.5%, 96.3% and 64.8% for Cd2+, Co2+, Cr3+ and Ni2+ respectively.70 | ||
| Fig. 1 Absorption capacity of EPS of B. thuringiensis PW-05 in presence of different concentration of mercury chloride supplement with respect to time of incubation i.e. 30, 60, 90, 120 min and 24 h. | ||
Spectroscopic analyses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which is similar to that of the result obtained by Qin et al.76 The other signals in the range of 4.0 to 3.4 ppm represent the protons in the sugar skeleton. It has been observed that certain peaks developed between 2.3 and 1.8 ppm after interaction between EPS and mercury was not found in pristine EPS and HgCl2. This phenomenon is attributed to the decrease in electron density around oxygen atoms and increase of electron density around hydrogen atoms of hydroxyl group.77
1 which is similar to that of the result obtained by Qin et al.76 The other signals in the range of 4.0 to 3.4 ppm represent the protons in the sugar skeleton. It has been observed that certain peaks developed between 2.3 and 1.8 ppm after interaction between EPS and mercury was not found in pristine EPS and HgCl2. This phenomenon is attributed to the decrease in electron density around oxygen atoms and increase of electron density around hydrogen atoms of hydroxyl group.77
Langmuir isotherm
The Langmuir isotherm is the widely applied theoretical model for monolayer adsorption. According to this model, adsorption occurs uniformly on the active sites of the adsorbent (EPS, in this case) and once an adsorbate (Hg2+) occupies a site, no further adsorption can take place at this site.41 Thus, the Langmuir equation is given by the following equation:
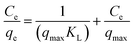 | (2) |
The Langmuir plots for EPS and Hg interaction at different temperatures are given in Fig. 5a. The isotherm parameters obtained at different temperatures are compiled in Table 2. The increase in qmax values with an increase in temperature is attributed to the fact that higher temperatures cause an enhancement in the sorption sites of the EPS. This favours the diffusion of the Hg2+ into the EPS matrix easily. The decrease in KL values with increasing the temperature indicates the lower heat of adsorption. This specifies that the interaction phenomenon is exothermic in nature. A further analysis of the Langmuir equation can be made on the basis of a dimensionless equilibrium parameter, RL, also known as the separation factor.83 This is given by:
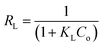 | (3) |
| Temp. (K) | KL (× 103 l g−1) | qmax | R2 | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1) |
|---|---|---|---|---|---|---|
| 298 | −0.208 | 0.0230 | 0.966 | −2.518 | −61.77 | |
| 308 | −0.192 | 0.0378 | 0.986 | −1.469 | −20.927 | −63.17 |
| 318 | −0.032 | 0.0420 | 0.991 | −1.293 | −61.74 |
Thermodynamic parameters for the interaction between EPS and Hg
To evaluate the thermodynamic aspects of the interaction of Hg with EPS, the thermodynamic parameters were calculated and are summarized in Table 2. From the values obtained for KL at different temperatures, the values of the Gibbs energy of interaction (ΔG) and the corresponding standard enthalpy (ΔH) and the standard entropy (ΔS) changes were obtained from the following equations:ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL KL |
A plot of ΔG/T versus 1/T provides a straight line, the slope of which is equal to ΔH. The negative values of ΔG for the interaction between Hg and EPS indicate both the feasibility and the spontaneity of the process. With increase in temperature, the ΔG value becomes less negative which means the efficient binding of Hg to EPS is more preferred at room temperature. The standard enthalpy change (ΔH) was found to be negative suggesting the exothermic nature of the process. The interaction process is accompanied by negative entropy which implies the increased order of the system upon binding of Hg onto EPS. The ΔG is regarded as the sum of enthalpic and entropic contributions. Considering the values of the thermodynamic parameters (Table 2), it is concluded that the interaction of Hg with EPS is an enthalpy-driven process.
4. Implications of this work
EPS and other biopolymers have been found to exhibit excellent metal binding properties with varied degrees of specificity and affinity.11 The binding of metal ions to bacterial biopolymer occurs through electrostatic interaction with the negatively charged functional groups such as uronic acids and phosphoryl groups,84 which has been well established in the present study for mercury. Additionally, EPS have impacts on the speciation and toxicity of mercury in the environment through metal mobilization and dispersion.40 Thus, bacterial EPS can be used as potential polymer for the bioremediation of Hg from toxic environments. The binding of mercury to EPS also affects its bioavailability and mobility and the high binding strength benefits for the recovery of metal ion from the EPS. Further, the bioavailability and mobility can reduce the risk of metal contamination in the waste water treatment practices. Hg after interacting with EPS in the environment can be recovered by complexation, ion exchange or surface precipitation.85 On the other hand, the bound mercury may be bioavailable to the bacteria harbouring mer operon in its genome. In this case, the Hg2+ can be converted to the volatile form of mercury i.e. Hg0 at a higher level to produce the less toxic form of mercury (Fig. 6).5. Conclusions
In this study, the interaction mechanism between bacterial EPS and mercury as a representative of the toxic metals have been explored and protein constituents of the EPS was found to be active participants responsible for the binding of mercury. The major functional groups in the interaction process include carboxyl, phosphoryl, hydroxyl, amino and sulfhydryl. Thus, bacterial EPS can be considered as a suitable agent for the treatment and recovery of mercury ions from contaminated wastes. Negative free energy demonstrates the enthalpy driven process of EPS–Hg interaction which is a thermodynamically favourable condition for waste water treatment and metal recovery practice. The increase in crystalline nature of EPS upon binding with mercury helps in the easy removal of metal associated EPS from the environment. Thus, EPS mediated removal of mercury is a promising alternative to the current metal removal practices.Acknowledgements
The authors of this manuscript declare that there is no conflict of interest. Authors would like to acknowledge the authorities of NIT, Rourkela for providing facilities. H. R. D. gratefully acknowledges the receipt of research fellowship from Ministry of Human Resource Development, Government of India. Instrument facilities of Department of Chemistry and Department of Physics of NIT, Rourkela has been utilized and gratefully acknowledged.References
- J. C. Hansen and G. Danscher, Rev. Environ. Health, 1997, 12, 107–116 CrossRef CAS PubMed.
- H. R. Dash and S. Das, Int. Biodeterior. Biodegrad., 2012, 75, 207–213 CrossRef CAS.
- S. Booth and D. Zeller, Environ. Health Perspect., 2005, 113, 521–526 CrossRef CAS PubMed.
- J. K. King, J. E. Kostka, M. E. Frischer, F. M. Saunders and R. A. Jahnke, Environ. Sci. Technol., 2001, 35, 2491–2496 CrossRef CAS PubMed.
- G. P. Sheng, M. L. Zhang and H. Q. Yu, Colloids Surf., B, 2008, 62, 83–90 CrossRef CAS PubMed.
- H. C. Flemming, T. R. Neu and D. J. Wozniak, J. Bacteriol., 2007, 189, 7945–7947 CrossRef CAS PubMed.
- L. Yang, Y. Liu, H. Wu, N. Ho, S. Molin and Z. J. Song, Int. J. Oral Sci., 2011, 3, 74–81 CrossRef PubMed.
- P. V. Bhaskar and N. B. Bhosle, Environ. Int., 2006, 32, 191–198 CrossRef CAS PubMed.
- Z. Q. Zhang, S. Q. Xia, X. J. Wang, A. Yang, B. Xu, L. Chen, Z. L. Zhu, J. F. Zhao, N. Jaffrezic-Renault and D. Leonard, J. Hazard. Mater., 2009, 163, 279–284 CrossRef CAS PubMed.
- F. Morcillo, M. T. González-Muñoz, T. Reitz, M. E. Romero-González, J. M. Arias and M. L. Merroun, PLoS One, 2014, 9(3), e91305 Search PubMed.
- A. Pal and A. K. Paul, Indian J. Microbiol., 2008, 48, 49–64 CrossRef CAS PubMed.
- J. M. Tapia, J. A. Munoz, F. Gonzalez, M. L. Blazquez, M. Malki and A. Ballester, Water Sci. Technol., 2009, 59, 1959–1967 CrossRef CAS PubMed.
- Z. Wang, Z. Wu and S. Tang, Water Res., 2009, 43, 2504–2512 CrossRef CAS PubMed.
- L. Duana, W. Jiangc, Y. Song, S. Xia and S. W. Hermanowicz, Bioresour. Technol., 2013, 148, 436–442 CrossRef PubMed.
- B. M. Lee, H. S. Shin and J. Hur, Chemosphere, 2013, 90, 237–244 CrossRef CAS PubMed.
- Z. Zhang and J. Zhang, Water, Air, Soil Pollut., 2014, 225, 1850–1860 CrossRef.
- Y. Liu, M. C. Lam and H. H. Fang, Water Sci. Technol., 2001, 43, 59–66 CAS.
- X. Wei, L. Fang, P. Cai, Q. Huang, H. Chen, W. Liang and X. Rong, Environ. Pollut., 2011, 159, 1369–1374 CrossRef CAS PubMed.
- T. C. Lau, X. A. Wu, H. Chua, P. Y. Qian and P. K. Wong, Water Sci. Technol., 2005, 52, 63–68 CAS.
- M. Ueshima, B. R. Ginn, E. A. Haack, J. E. S. Szymanowski and J. B. Fein, Geochim. Cosmochim. Acta, 2008, 72, 5885–5895 CrossRef CAS.
- X. Pan, J. Liu, W. Song and D. Zhang, Front. Environ. Sci. Eng., 2012, 6, 493–497 CrossRef CAS.
- H. R. Dash, N. Mangwani, J. Chakraborty, S. Kumari and S. Das, Appl. Microbiol. Biotechnol., 2013, 97, 561–571 CrossRef CAS PubMed.
- A. Poli, G. Anzelmo and B. Nicolaus, Mar. Drugs, 2010, 8, 1779–1802 CrossRef CAS PubMed.
- M. S. Quigley, P. H. Santschi, C. C. Hung, L. Guo and B. D. Honeyman, Mar. Chem., 2002, 76, 27–45 CrossRef.
- D. L. Hoskins, S. E. Stancyk and A. W. Decho, Mar. Ecol.: Prog. Ser., 2003, 247, 93–101 CrossRef.
- S. H. Ko, H. S. Lee, S. H. Park and H. K. Lee, Biotechnol. Bioprocess Eng., 2000, 5, 81–185 Search PubMed.
- M. A. Kumar, K. T. K. Anandapandian and K. Parthiban, Braz. Arch. Biol. Technol., 2011, 54, 259–265 CrossRef CAS.
- W. Zhang, L. Chen and D. Liu, Appl. Microbiol. Biotechnol., 2012, 93, 1305–1314 CrossRef CAS PubMed.
- H. R. Dash, N. Mangwani and S. Das, Environ. Sci. Pollut. Res., 2014, 21, 2642–2653 CrossRef CAS PubMed.
- P. D'Abzac, F. Bordas, E. Joussein, E. van Hullebusch, P. L. Lens and G. Guibaud, Environ. Sci. Pollut. Res., 2012, 20, 4509–4519 CrossRef PubMed.
- N. Das and P. Karthika, Indian J. Biotechnol., 2008, 7, 159–169 CAS.
- I. D. S. Henriques and N. G. Love, Water Res., 2007, 41, 4177–4185 CrossRef CAS PubMed.
- M. G. Baker, S. V. Lalonde, K. O. Konhauser and J. M. Foght, Appl. Environ. Microbiol., 2010, 76, 102–109 CrossRef CAS PubMed.
- T. Smitinont, C. Tansakul, S. Tanasupawat, S. Keeratipibul, L. Navarini, M. Bosco and P. Cescutti, Int. J. Food Microbiol., 1999, 51, 105–111 CrossRef CAS PubMed.
- J. Chakraborty and S. Das, Environ. Sci. Pollut. Res., 2014, 21, 14188–14201 CrossRef CAS PubMed.
- F. A. Loewus, Anal. Chem., 1952, 24, 219 CrossRef CAS.
- B. Frolund, R. Palmgren, K. Keiding and P. H. Nielsen, Water Res., 1996, 30, 1749–1758 CrossRef.
- S. B. Subramaniam, S. Yan, R. D. Tyagi and R. Y. Surampalli, Water Res., 2007, 12, 1–7 Search PubMed.
- P. Desjardins and D. Conklin, J. Visualized Exp., 2010, 2565 Search PubMed.
- G. P. Sheng, J. Xu, H. W. Luo, W. W. Li, W. H. Li, H. Q. Yu, Z. Xie, S. Q. Wei and F. C. Hu, Water Res., 2013, 47, 607–614 CrossRef CAS PubMed.
- R. Gong, J. Ye, W. Dai, X. Yan, J. Hu, X. Hu, S. Li and H. Huang, Ind. Eng. Chem. Res., 2013, 52, 14297–14303 CrossRef CAS.
- F. François, C. Lombard, J. M. Guigner, P. Soreau, F. B. Jaisson, G. Martino, M. Vandervennet, D. Garcia, A. L. Molinier, D. Pignol, J. Peduzzi, S. Zirah and S. Rebuffat, Appl. Environ. Microbiol., 2012, 78, 1097–1106 CrossRef PubMed.
- J. F. Santos-Gandelman, M. Giambiagi-deMarval, G. Muricy, T. Barkay and M. S. Laport, Antonie van Leeuwenhoek, 2014, 106, 585–590 CrossRef CAS PubMed.
- M. A. Smith and M. J. Bidochka, Can. J. Microbiol., 1998, 44, 351–355 CrossRef CAS PubMed.
- G. Spengler, A. Molnár, Z. Schelz, L. Amaral, D. Sharples and J. Molnár, Curr. Drug Targets, 2006, 7, 823–841 CrossRef CAS PubMed.
- A. Beceiro, M. Tomás and G. Bou, Clin. Microbiol. Rev., 2013, 26, 185–230 CrossRef CAS PubMed.
- C. Kunacheva and D. C. Stuckey, Water Res., 2014, 61, 1–18 CrossRef CAS PubMed.
- I. Dogsa, M. Kriechbaum, D. Stopar and P. Laggner, Biophys. J., 2005, 89, 2711–2720 CrossRef CAS PubMed.
- B. Vu, M. Chen, R. J. Crawford and E. P. Ivanova, Molecules, 2009, 14, 2535–2554 CrossRef CAS PubMed.
- P. V. Bhaskar and N. B. Bhosle, Curr. Sci., 2005, 88, 45–53 CAS.
- K. Kavita, A. Mishra and B. Jha, Biofouling, 2011, 27, 309–317 CrossRef CAS PubMed.
- K. Kavita, V. K. Singh, A. Mishra and B. Jha, Carbohydr. Polym., 2014, 101, 29–35 CrossRef CAS PubMed.
- Y. Jiao, G. D. Cody, A. K. Harding, P. Wilmes, M. Schrenk, K. E. Wheeler, J. F. Banfield and M. P. Thelen, Appl. Environ. Microbiol., 2010, 76, 2916–2922 CrossRef CAS PubMed.
- R. Mikuttaa, U. Zang, J. Chorover, L. Haumaier and K. Kalbitz, Geochim. Cosmochim. Acta, 2011, 75, 3135–3154 CrossRef.
- S. Tsuneda, H. Aikawa, H. Hayashi, A. Yuasa and A. Hirata, FEMS Microbiol. Lett., 2003, 223, 287–292 CrossRef CAS PubMed.
- K. Jain, S. Parida, N. Mangwani, H. R. Dash and S. Das, Ann. Microbiol., 2013, 63, 1553–1562 CrossRef CAS.
- G. O. Phillips and P. A. Williams, Handbook of Hydrocolloids, Woodhead Publishing Limited, 2000.
- N. Staats, B. De Winder, L. Stal and L. Mur, Eur. J. Phycol., 1999, 34, 161–169 CrossRef.
- G. Sheng, H. Yu and Z. Yu, Appl. Microbiol. Biotechnol., 2005, 67, 125–130 CrossRef CAS PubMed.
- K. K. T. Goh, D. R. Haisman and H. Singh, Appl. Microbiol. Biotechnol., 2005, 67, 202–208 CrossRef CAS PubMed.
- A. Omoike and J. Chorover, Biomacromolecules, 2004, 5, 1219–1230 CrossRef CAS PubMed.
- E. Dertli, M. J. Mayer and A. Narbad, BMC Microbiol., 2015, 15, 8 CrossRef PubMed.
- K. Tsuzuki, M. Sugiyama and N. Haramaki, Environ. Health Perspect. Suppl., 1994, 102, 341–342 CrossRef CAS PubMed.
- H. Miyachi, T. Matsui, Y. Shigeta and K. Hirao, Phys. Chem. Chem. Phys., 2010, 12, 909–917 RSC.
- M. H. Shamsi and H. Kraatz, J. Inorg. Organomet. Polym., 2013, 23, 4, DOI:10.1007/s10904-012-9694-8.
- F. Schurz, M. Sabater-Vilar and J. Fink-Gremmels, Mutagenesis, 2010, 15, 525–530 CrossRef.
- S. P. Mohapatra, M. A. Siebel and G. J. Alaerts, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 1995, 28, 615–629 CrossRef.
- J. H. Chen, L. W. Lion and W. C. Ghiorse, Water Res., 1995, 29, 421–430 CrossRef CAS.
- G. Mandal, S. Bhattacharya and T. Ganguly, Chem. Phys. Lett., 2009, 478, 271–276 CrossRef CAS.
- Y. Tian, L. Zheng and D. Z. Sun, J. Environ. Sci., 2006, 18, 420–427 CAS.
- Q. Q. Yang, J. G. Liang and H. Y. Han, J. Phys. Chem. B, 2009, 113, 10454–10458 CrossRef CAS PubMed.
- J. Schmitt and H. Flemming, Int. Biodeterior. Biodegrad., 1998, 41, 1–11 CrossRef CAS.
- F. Szurdoki, H. Kido and D. Hammock, Bioconjugate Chem., 1995, 6, 145–149 CrossRef CAS PubMed.
- R. K. Zalups, Pharmacol. Rev., 2013, 52, 113–143 Search PubMed.
- C. Song, X. F. Sun, S. F. Xing, P. F. Xia, Y. J. Shi and S. G. Wang, Environ. Sci. Pollut. Res., 2014, 21, 1786–1795 CrossRef CAS PubMed.
- G. Qin, L. Zhu, X. Chen, P. G. Wang and Y. Zhang, Microbiology, 2007, 153, 1566–1572 CrossRef CAS PubMed.
- M. Balci, Basic 1H and 13C-NMR Spectroscopy, Elsevier, 2005, ISBN: 978-0-444-51811-8 Search PubMed.
- R. Yu, J. Tan, P. Yang, J. Sun, Y. Ou, J. Xiong and Y. Dai, Trans. Nonferrous Met. Soc. China, 2008, 18, 1427–1432 CrossRef CAS.
- S. S. Khan, E. B. Kumar, A. Mukherjee and N. Chandrasekaran, J. Basic Microbiol., 2010, 51, 183–190 CrossRef PubMed.
- R. Ahmad and Q. Faisal, Curr. World Environ., 2011, 6, 115–125 CAS.
- C. K. Gupta and T. K. Mukherjee, Hydrometallurgy in Extraction Processes, CRC Press, 1990, vol. 1, p. 248 Search PubMed.
- Y. Sun, L. Zhu, T. Wu, T. Cai, E. M. Gunn and L. Yu, AAPS J., 2012, 14, 380–388 CrossRef CAS PubMed.
- E. Oguz, Colloids Surf., B, 2005, 252, 121–128 CrossRef CAS.
- M. Santamaria, A. R. Diaz-Marreto, J. Hernandez, A. M. Uutierrez-Navarro and J. Corzo, Environ. Microbiol., 2003, 5, 916–924 CrossRef CAS PubMed.
- W. B. Beech and J. Sunner, Curr. Opin. Biotechnol., 2004, 15, 181–186 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |