A new type of SO3H-functionalized magnetic-titania as a robust magnetically-recoverable solid acid nanocatalyst for multi-component reactions†
Abstract
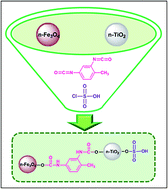
SO3H-functionalized magnetic-titania nanoparticles (Fe3O4@TDI@TiO2–SO3H) have been synthesized by a two-step procedure, involving the covalent grafting of n-TiO2 to n-Fe3O4 via 2,4-toluene diisocyanate as a regioselective linker (n-Fe3O4@TDI@TiO2) and subsequent sulfonation using chlorosulfonic acid. The as-prepared nanocatalyst was characterized by Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) and vibrating sample magnetometry (VSM). The catalytic activity of the nanocatalyst was assessed for the synthesis of benzimidazoquinazolinones and polyhydroquinolines, in which the reaction conditions were optimized by applying central composite design (CCD) through response surface methodology. The nanocatalyst could be separated from the reaction mixture easily by magnetic decantation and reused at least six times without a noticeable degradation in catalytic activity. To the best of our knowledge, there are no literature reports on applying experimental design to optimize the reaction conditions for benzimidazoquinazolinones synthesis.


 Please wait while we load your content...
Please wait while we load your content...