Dual enzymatic dynamic kinetic resolution by Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase and Candida antarctica lipase B†
Abstract
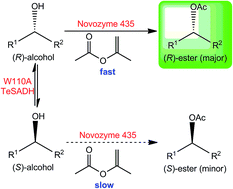
The immobilization of Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase (TeSADH) using sol–gel method enables its use to racemize enantiopure alcohols in organic media. Here, we report the racemization of enantiopure phenyl-ring-containing secondary alcohols using xerogel-immobilized W110A TeSADH in hexane rather than the aqueous medium required by the enzyme. We further showed that this racemization approach in organic solvent was compatible with Candida antarctica lipase B (CALB)-catalyzed kinetic resolution. This compatibility, therefore, allowed a dual enzymatic dynamic kinetic resolution of racemic alcohols using CALB-catalyzed kinetic resolution and W110A TeSADH-catalyzed racemization of phenyl-ring-containing alcohols.


 Please wait while we load your content...
Please wait while we load your content...