The effect of spermidine on the structure, kinetics and stability of proteinase K: spectroscopic and computational approaches
Abstract
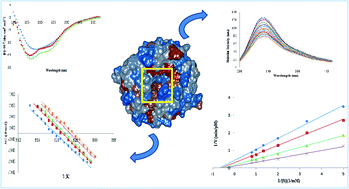
Polyamines (such as spermidine) are low molecular weight compounds which can be used as cosolvents in biological and industrial applications. Cosolvents can interact with proteins and alter their stability and structure. We aimed to study the effect of spermidine (Spd) interaction with proteinase K (PK), with respect to its industrial and biological applications, using different spectroscopic methods of fluorescence, circular dichroism (CD) and UV-visible (UV-vis), as well as simulation methods. Fluorescence quenching data analysis demonstrated that PK had one binding site for Spd. The reduction of Stern–Volmer constant values by increasing the temperature suggested that binding between enzyme and Spd occurred in the ground state (static quenching) and the stability of the complex was decreased at higher temperatures. The values of enthalpy and entropy changes as well as simulation methods illustrated that hydrogen bonds and van der Waals forces played a major role in the PK–Spd complex formation. CD data analysis also showed the decrease in the β-sheet and the increase in the α-helix and β-turn of PK induced by Spd. UV-visible results indicated some alteration in the hydrophobicity of the Trp microenvironment of PK by adding Spd, confirming the intrinsic fluorescence emission results. The kinetic and thermal stability studies showed that Spd could increase the activity and stability of PK, and that this might be due to the changes in the secondary and tertiary structures of the enzyme after Spd modification.

 Please wait while we load your content...
Please wait while we load your content...