Structural investigation of calcium borosilicate glasses with varying Si/Ca ratios by infrared and Raman spectroscopy
Abstract
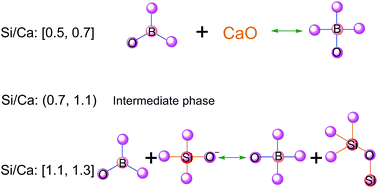
Calcium borosilicate glasses with varying Si/Ca ratios (with constant Si/B value) have been successfully prepared using a normal melt-quench technique. Structural features were investigated through infrared (IR) spectroscopy, Raman spectroscopy and differential thermal analysis (DTA). The IR and Raman spectra showed a Si/Ca ratio-dependent structure of borosilicate, which can provide valuable information to predict the composition and structure relations in the glasses. Conversion of BO3 to BO4 units was driven mainly by the disproportionation reaction between BO3 units and CaO when 0.5 ≤ Si/Ca ≤ 0.7. However, the BO3 units captured the non-bridging oxygens of Q1 units when 1.1 ≤ Si/Ca ≤ 1.3, resulting in the formation of more BO4 and Si–O–Si bonds. Qn (n = 0, 1, 2, 3, 4) notation was used to represent the SiO4 tetrahedral units, where n is the number of bridging oxygen per SiO4 unit. The intermediate phase was presented when 0.7 < Si/Ca < 1.1. Variations in density, glass transition temperature and thermal stability as functions of Si/Ca ratios correspond to the change in BO4, BO3 and SiO4 structural units. The intermediate phase results in maximum density, minimum molar volumes and maximum Tg, which can be expected to be the optimum candidate for applications.

 Please wait while we load your content...
Please wait while we load your content...