Analyte-concentrating 3D hybrid plasmonic nanostructures for use in highly sensitive chemical sensors†
MinKyoung Lee‡
ab,
ChaeWon Mun‡a,
Dong-Ho Kim*a,
Seung-Cheol Chang*b and
Sung-Gyu Park*a
aAdvanced Functional Thin Films Department, Korea Institute of Materials Science (KIMS), Changwon, Gyeongnam 641-831, Korea. E-mail: dhkim2@kims.re.kr; sgpark@kims.re.kr
bInstitute of BioPhysio Sensor Technology, Graduate Department of Molecular Science Technology, Pusan National University, Busan 46241, Korea. E-mail: s.c.chang@pusan@ac.kr
First published on 22nd September 2016
Abstract
We investigated the analyte-concentrating effects of 3D porous Ag hybrid nanostructures that displayed superhydrophobicity toward aqueous solutions and the coffee ring effect toward organic solutions in an effort to develop highly sensitive SERS-based chemical sensors. The 3D hybrid nanostructures were composed of 3D-stacked Ag nanowires (NWs) and nanoparticles (NPs) separated by an alumina interlayer that enhanced plasmonic coupling between the high-density Ag nanomaterials. The antiwetting properties of the 3D plasmonic nanostructures were provided by the specific chemisorption of 1H,1H,2H,2H-perfluorodecanethiol (PFDT) onto the Ag nanomaterials. Synergy between the analyte-enriching effects due to the antiwetting properties and matching of the localized surface plasmon resonance (LSPR) wavelength and the excitation laser wavelength yielded a superhydrophobic 3D porous SERS platform that enabled the ultrasensitive detection of methylene blue in an aqueous solution with a limit of detection (LOD) of 0.15 pM, 104-fold lower than the value obtained from the as-prepared hydrophilic counterparts. Both the PFDT-grafted and as-prepared SERS substrates showed complete wetting with a contact angle (CA) of 0° for organic liquids (i.e., low surface tension liquids), such as acetone. An alternative analyte-concentrating strategy is, therefore, needed for organic solutions. Interestingly, an acetone solution into which had been dissolved a pesticide (iprodione) exhibited a dark ring-shaped concentrated deposit after liquid evaporation, normally known as the coffee ring effect. The SERS intensity line profiles of the iprodione molecules exhibited a 5.7-fold signal enhancement at the ring edge compared to the ring center. The detection of toxins in dissolved organic liquids may be achieved using the coffee ring effect of the 3D porous SERS platform to enhance the SERS sensitivity with an LOD of 250 ng for iprodione molecules. This LOD was 102-fold lower than the European regulatory limits (20 μg/1 kg fruits).
Introduction
Surface-enhanced Raman spectroscopy (SERS) exploits strong electromagnetic (EM) fields in nanoscale gaps (or hot spots) between metallic nanostructures as a result of coupled localized surface plasmon resonance (LSPR) excitation.1–4 The total power (ISERS) of the SERS radiation from the analyte molecules adsorbed onto the plasmonic nanostructures may be described as:| ISERS ∝ N·σSERS·|Eloc|4/|E0|4·|E0|2 | (1) |
Once high-performance plasmonic nanostructures have been fabricated, the next step will require the development of a sampling method to further decrease the limit of detection (LOD) (i.e., increase N for a given molar concentration in eqn (1)). The LOD is normally reduced by reducing the wetted area of an analyte solution drop. This is accomplished by using plasmonic nanostructures with antiwetting surface properties.14–17 Recently, 3D stacked mesh-like Ag NWs have been assembled in a layer-by-layer manner, and subsequent chemical functionalization of the Ag NW stacks has provided omniphobic SERS substrates for use in the detection of toxins dissolved in either water or organic liquids.16 Another analyte-concentrating strategy relies on the coffee ring effect. The coffee ring effect results from the outward capillary flow within a solution droplet during evaporation of the solvent under three-phase contact line pinning.18–25 Non-volatile solutes, such as organic small molecules, biomacromolecules, and nanoparticles, are thereby concentrated at the periphery of the original droplet, resulting in a ring-shaped concentrated deposit. Based on the coffee-ring effect, highly efficient SERS platforms which integrate the aggregation of metal nanoparticles (or nanowires) and the enrichments of analytes have been realized.18–20
In the present work, to enhance the SERS sensitivity, we developed a method for enriching analytes on 3D porous hybrid Ag nanostructures based on superhydrophobicity toward aqueous solutions and the coffee ring effect toward organic solutions. The large-area (4 cm in diameter) plasmonic nanostructures were composed of 3D-stacked Ag NWs and NPs separated by a 10 nm thick alumina interlayer that boosted plasmonic coupling between the ultrahigh-density Ag nanomaterials.3 The antiwetting properties of the 3D hybrid Ag nanostructures were obtained by introducing chemical fluorination onto the nanostructure surfaces using 1H,1H,2H,2H-perfluorodecanethiol (PFDT). The initial water contact angle (CA) was 159°, providing a small wetted solid fraction of water on the 3D nanostructured surface. A wetted solid fraction of 0.10 indicated that water did not penetrate the 3D porous network, and a water droplet could sit on the top region of the 3D stacked porous nanostructures. We observed that in the case of the as-prepared hydrophilic counterparts, a water droplet readily spread out randomly over 3 dimensions (in the x–y directions and in the z-direction), and water fully penetrated the back side of the porous glass fiber substrate. The self-assembled monolayer (SAM) of the PFDT on the Ag nanomaterials tuned the LSPR frequency of the 3D plasmonic nanostructures. The as-prepared 3D hybrid Ag nanostructures displayed an LSPR peak wavelength of 500 nm, and the LSPR wavelength of the 3D hybrid Ag nanostructures was red-shifted near 630 nm due to the local refractive index (RI) change at the adsorbed PFDT (RI = 1.333). The PDFT-grafted 3D hybrid Ag nanostructures were expected to provide a better SERS sensitivity upon laser excitation at 632.8 nm to provide Raman measurements. The synergy effects of the analyte-enrichment and optimization of the LSPR wavelength enabled the superhydrophobic 3D porous SERS platform to ultrasensitively detect methylene blue (MB) in an aqueous solution with an LOD of 0.15 pM, 104-fold lower than the LOD of the hydrophilic as-prepared counterparts.
The SERS detection of toxins dissolved in organic liquids having a low surface tension was achieved through the use of the coffee ring effect, which concentrated the analyte. To the best of our knowledge, use of the coffee ring effect on a 3D porous SERS substrate has not been realized previously. We selected iprodione as the probe molecule. Iprodione is an agricultural pesticide and is considered to be highly hazardous with a maximum residue level (MRL) of 20 ppb for fruits.26 The solubility of iprodione in water is extremely low, 12.2 mg L−1, and the solubility in organic liquids, such as acetone, is high (324 g L−1). Due to the high solubility of toxins such as iprodione in organic liquids, such liquids are typically used to extract toxins from crops during food safety monitoring. We found that the iprodione deposits were highly concentrated at the periphery of the original droplet on the 3D porous SERS substrate. The SERS intensity line profiles of the iprodione molecules exhibited a 5.7-fold signal enhancement at the ring edge compared to the ring center. The enrichment of the analytes by the coffee ring effect on the 3D hybrid Ag nanostructured surface provided an LOD of 250 ng iprodione.
Results and discussion
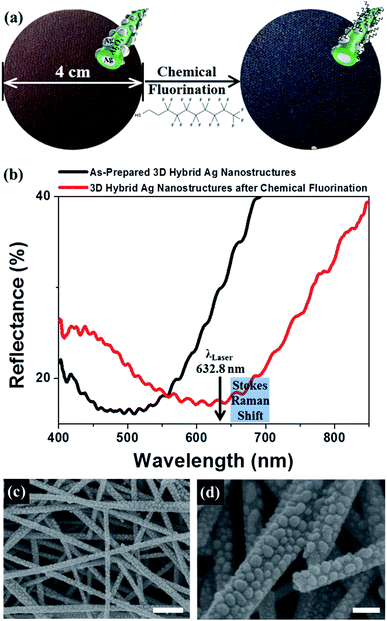
Fig. 1a presents reflection photographs of large-area (4 cm in diameter) 3D porous SERS substrates prepared using vacuum filtration of Ag NW inks over a glass microfiber filter and thermal evaporation of an alumina interlayer and Ag NPs layers onto 3D stacked Ag NWs/glass fiber substrates. The fabrication process is described in detail elsewhere.3 The as-prepared 3D hybrid Ag nanostructures reflected a uniform brown color that changed to navy upon vapor treatment with PFDT. The specific affinity of the alkanethiols for the Ag nanomaterials produced self-assembled monolayers (SAMs) of PFDT on the Ag nanoparticles,17,27,28 leading to changes in the optical properties of the plasmonic nanostructures. Fig. 1b shows the corresponding reflectance spectra of the 3D hybrid Ag nanostructures as a function of chemical fluorination. The as-prepared 3D hybrid Ag nanostructures showed a broad LSPR peak wavelength of 500 nm, and the LSPR peak position of the 3D hybrid Ag nanostructures was red-shifted near 630 nm due to the local refractive index (RI) change in the adsorbed PFDT (RI = 1.333 at 589.9 nm). The PFDT-grafted 3D hybrid Ag nanostructures, therefore, are expected to provide a better SERS sensitivity upon laser excitation at 632.8 nm for Raman measurements due to agreement between the LSPR wavelength and the laser excitation wavelength. Specifically, excitation at an LSPR wavelength that is intermediate between the excitation and Raman wavelength (that is, a wavelength exceeding the excitation wavelength) yielded the strongest SERS signal.29 We confirmed that the surface morphology remained unchanged upon PFDT vapor treatment, as shown in Fig. 1c and d. A Ag evaporation thickness of 11 nm produced high-density Ag NPs with ∼330 μm−2 that decorated the large surfaces of the 10 nm-thick alumina-coated 3D-stacked Ag nanowires. The ultrahigh density of the Ag NWs and NPs enhanced the plasmonic coupling at the air nanogaps between Ag NPs and at the 10 nm thick alumina interlayer between the Ag NPs and the Ag NWs.3Before investigating analyte-concentrating effects of 3D porous Ag hybrid nanostructures, we calculated the enhancement factor (EF) of as-prepared 3D SERS substrates (see ESI† for details of the EF calculation). We obtained the Raman intensities of the monolayer of benzenethiol (BT) on the surface of 3D Ag nanostructures, ISERS, and of pure bulk BT solution, IRaman (Fig. S1 in the ESI†). The estimated number of BT molecules for both measurements were designated as NSERS and NRaman, respectively. The EF values based on the Raman intensity of BT at 998 cm−1 and 1015 cm−1 are 4.6 × 106 and 1.2 × 107, respectively. These values are comparable to previously reported values.8,18–20,30
The antiwetting properties of the fluorine-functionalized 3D porous Ag nanostructures were characterized by applying a water droplet with volume of 8 μL onto the 3D hybrid nanostructures, as shown in Fig. 2a. It should be noted that it was difficult to apply a water droplet onto the very slippery 3D porous networks (Video 1 in the ESI†). The water drop assumed a spherical shape with a CA of 159°. The superhydrophobic behavior could be expressed in terms of the Cassie–Baxter equation,31–33
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θr = f(1 + cos θr = f(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θs) − 1 θs) − 1
| (2) |
Fig. 2b plots the time-dependent variations of the water CA after applying a water droplet to the superhydrophobic SERS substrate. During droplet shrinkage, the CA and wetted area of the drop varied. The water CA gradually decreased from 159° to 144° over 10 min. The angle then remained constant, above 120°, until 80 min. Finally, the water was completely evaporated after 100 min. The left inset image in Fig. 2b shows the analyte enrichment effect as a result of the antiwetting properties of the 3D porous network. Under wetting conditions, water spread in the horizontal direction and penetrated vertically into the 3D porous nanostructures within a few seconds (right inset image in Fig. 2b and Video 2 in the ESI†). We observed that on the as-prepared hydrophilic 3D porous structures, a water droplet spread in 3 dimensions (in the x–y direction and in the z direction), and the water droplet fully penetrated the back side of the porous glass fiber substrate.
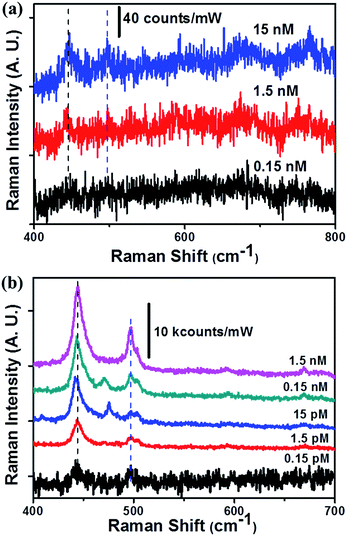
The analyte-concentrating effects, which enhanced the detection sensitivity on the 3D porous SERS substrates, were investigated using an MB aqueous solution. Fig. 3 shows the surface-enhanced resonance Raman spectroscopy (SERRS) spectra collected at different concentrations, which depended on the surface fluorination. A very low power of 40 μW and a 100× objective lens were used to avoid generating a fluorescence background signal from the MB. Both hydrophilic and superhydrophobic SERS substrates showed major MB Raman bands at 445 and 497 cm−1 that were assigned to the C–N–C skeletal bending of the MB molecules.34,35 The LOD for the hydrophilic 3D Ag nanostructures was measured to be 1.5 nM (at this time, we measured the LOD at the central point of the initial droplet), similar to previous results.3 The superhydrophobic SERS substrates enhanced the LOD value 104-fold. This enhancement in the LOD of 0.15 pM could be explained as resulting from two factors: the analyte-concentrating effects and the tuning of the LSPR frequency through surface functionalization. The aqueous solution could be diluted in all directions of the 3D porous networks on the wetted porous structures. We observed the water-wetted region on the backside of the 3D SERS substrates (0.2 μm thick 3D stacked Ag NWs and 420 μm thick glass microfibers) upon application of 10 μL of an MB solution. A 2.1 × 103-fold analyte dilution occurred along the z-direction, assuming a laser penetration depth of 0.2 μm.36 In the x–y plane, we benefitted from a 4-fold concentration increase (based on the wetted diameter on the hydrophilic and hydrophobic SERS substrates; (4 mm/2 mm)2 = 4) due to the antiwetting properties. The LSPR wavelength tuning due to the chemisorption of PFDT was also characterized. The LSPR wavelength (about 630 nm) appeared to match the laser excitation wavelength (632.8 nm), and the Stokes Raman shift (longer than 632.8 nm) reduced the LOD value.
Replacing water, which has a high surface tension of 72.80 mN m−1, with acetone, which has a low surface tension of 25.20 mN m−1, resulted in complete wetting properties on both the as-prepared and PFDT-treated 3D porous SERS substrates (see Video 3 in the ESI†) on the 3D nanostructures with high porosity. Unlike the multi-layered Ag NW mesh structures, the fluorine-grafted 3D Ag hybrid nanostructures did not show oleophobic properties (i.e., the CA of toluene, which has a surface tension of 28.40 mN m−1, was 111°). The multi-layered Ag NW structures comprised aligned high-density Ag NWs, which reduced the porosity compared to the Ag NW hybrid nanostructures. A re-entry geometry may potentially be formed in the layer-by-layer assembly of Ag NW stacks to produce oleophobicity.8,37
Acetone-soluble pesticide detection was achieved using the untreated 3D Ag nanostructures as SERS substrates and iprodione as the probe molecule. Iprodione is an agricultural pesticides and is considered to be a highly hazardous chemical with a maximum residue level (MRL) of 20 ppb for fruits.26 Since the iprodione solubility (12.2 mg L−1) in water is extremely low, organic liquids with a high solubility for iprodione (324 g L−1), such as acetone, may be used to extract iprodione from crops for food safety monitoring. We applied a 5 μL drop of a 1 mg mL−1 iprodione acetone solution onto the as-prepared 3D SERS substrates. Interestingly, very dark concentric ring-shaped deposits were observed after acetone evaporation, as shown in Fig. 4a. Isotropic spreading by the randomly oriented Ag NW networks produced circular deposits of non-volatile iprodione molecules. Ring-shaped deposits could be explained in terms of the classical coffee ring effect.21–25 The three-phase contact lines on the 3D porous surface were pinned, and iprodione molecules were transported to the perimeter due to the outward capillary flow inside the 3D porous network (Fig. 4b). Finally, highly concentrated deposits of iprodione formed at the perimeter of the porous medium.
We investigated the concentration distribution of analytes across the ring-shaped deposit. Iprodione is characterized by a major peak at 1000 cm−1, which corresponds to a benzene breathing mode (Fig. S3 in the ESI†). This characteristic signature of iprodione is clearly visible throughout the line profiles obtained at 0.1 mm steps, as shown in Fig. 5a. The arrow in the inset shows the line profiling direction from the ring center to the ring periphery. The SERS intensity measured at the ring edge (the average value of the blue area) was 5.7 times higher than that measured at the ring center (average value of the brown area), as shown in Fig. 5b. We also clearly found that the analytes distribution was not uniform in the ring zone due to the Marangoni flow generation during liquid evaporation (Fig. 5b).38 To obtain reliable quantification in the ring zone and further reproducible calibration plot for different analyte concentrations, it's better to apply SERS intensity area mapping in the ring zone or use large detection area for single point measurement. When we use laser wavelength of 632.8 nm with a 100× objective lens (NA = 0.9), the illumination area is calculated to be 0.58 μm2. We can obtain SERS intensity area mapping in the ring area. Then an average SERS intensity value measured for each analyte concentration can be used for calibration plot. Another method is to use one large illumination area to cover the entire ring region. Normally, portable Raman spectrometer employs large spot diameter with mm scale, then the illumination area can cover the entire ring zone (0.6 mm in length in our case, as shown in Fig. 5). Then we can obtain the averaged SERS intensity from the deposited molecules in the ring zone. We believe that obtaining reliable quantitative results is essential for practical SERS-based sensing applications.
The coffee ring effect greatly reduced the LOD of the pesticides. We dropped 5 μL of iprodione acetone solutions having different concentrations onto the 3D plasmonic nanostructures. A 5 μg iprodione deposit was created by dissolving 5 mg iprodione in 5 mL acetone, and 5 μL of the iprodione solution were dropped onto the 3D plasmonic nanostructures. Fig. 6a shows a comparison of the SERS spectra obtained at 25 μg or 50 ng iprodione deposit after evaporation of the acetone. The SERS spectra were collected at the original drop edge at each concentration. The LOD of iprodione was determined to be 250 ng. The Raman intensity as a function of the final quantity of iprodione deposited was used to calibrate the measurement, as shown in Fig. 6b. Since the regulatory limit is 20 μg iprodione based on 1 kg fruits,26 the LOD of 250 ng for iprodione molecules clearly indicated that the 3D porous Ag SERS platform provided a highly sensitive SERS-based chemical sensor.
Conclusions
In summary, we developed an analytes-concentrating strategy on the 3D highly porous hybrid Ag nanostructures by utilizing both superhydrophobicity toward aqueous solutions and the coffee ring effect toward organic toxin solutions. The synergy between the analyte-enriching effects due to the antiwetting properties and the tuned optical properties provided a superhydrophobic 3D porous SERS platform for the ultrasensitive detection of methylene blue aqueous solutions with an LOD of 0.15 pM, 104-fold lower than the LOD of the hydrophilic as-prepared counterparts. We also investigated the analyte enrichment of an organic toxin solution using the coffee ring effect. The SERS intensity line profiles of the probe molecules revealed a 5.7-fold signal enhancement at the ring edge compared to the ring center. This effect was used to develop a highly sensitive SERS-based pesticide detection method with a pesticide LOD of 250 ng iprodione. Combined with the highly optimized plasmonic nanostructures and the analyte-concentrating effects, our 3D porous SERS platform offers an attractive chemical sensor for use in the fields of food and crop safety, illicit drug use testing, explosive detection, and forensic applications, in which trace analytes must be extracted and detected.Experimental
Fabrication of the 3D hybrid Ag nanostructures and chemical functionalization
The fabrication of the 3D hybrid Ag nanostructures is described in detail elsewhere.3 Briefly, the 3D hybrid Ag nanostructures were prepared from a 3D stack of Ag NWs and NPs separated by an alumina interlayer. The 3D stacked Ag NWs were formed by vacuum filtration over a 420 μm thick glass microfiber filter, and the alumina and Ag NPs were deposited onto the 3D-stacked Ag NWs by thermal evaporation. The nominal thicknesses of the Ag and alumina layers were monitored using a quartz crystal oscillator. The as-prepared 3D hybrid nanostructures were then functionalized with PFDT. Specifically, 4 μL of a 97% PFDT solution were poured into a glass Petri dish, and the lid of the Petri dish was closed for 2 hours. The lid was attached to the 3D SERS substrates or a smooth 100 nm thick Ag film. We used a 100 nm thick smooth Ag film as a control for comparison with the 3D hybrid Ag nanostructures. Water was used for the surface wettability measurements, and the Ag film exhibited a CA of 107°, similar to the CA reported previously.16Measurements and characterizations
CAs were measured using CA goniometry (FEMTOFAB, SDLab-200TEZ). The surface morphologies were characterized by field emission scanning electron microscopy (FE-SEM; Jeol JSM-6700F). The reflectance spectra of the 3D nanostructures were measured using spectrometers (Ocean Optics, USB4000) attached to an optical microscope (Nikon, L150) with a 20× objective lens. The light reflected from the 3D nanostructures was collected through the same objective lens and analyzed by spectrometry. The light reflected from the smooth silver mirror was measured as a reference. The normalized reflectance spectra of the plasmonic nanostructures are shown in Fig. 1b.SERS measurements were collected using a Raman microscope (Horiba Jobin Yvon) equipped with a spectrometer and a thermoelectrically cooled charge coupled device (CCD). The excitation laser had a wavelength of λ = 632.8 nm, and the power impinging on the sample was 0.04 mW. A 100× objective lens with NA = 0.9 was used to focus the excitation laser onto the sample and collect the scattered Raman signals.
Acknowledgements
This study was supported financially by the Fundamental Research Program (PNK4660) of the Korean Institute of Materials Science (KIMS). S.-G. Park is grateful for support from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A01053884).Notes and references
- D. K. Lim, K. S. Jeon, H. M. Kim, J. M. Nam and Y. D. Suh, Nat. Mater., 2010, 9, 60–67 CrossRef CAS PubMed.
- D. K. Lim, K.-S. Jeon, J.-H. Hwang, H. Kim, S. Kwon, Y. D. Suh and J.-M. Nam, Nat. Nanotechnol., 2011, 6, 452–460 CrossRef CAS PubMed.
- S.-G. Park, C. Mun, M. Lee, T. Y. Jeon, H.-S. Shim, Y.-J. Lee, J.-D. Kwon, C. S. Kim and D.-H. Kim, Adv. Mater., 2015, 27, 4290–4295 CrossRef CAS PubMed.
- S.-G. Park, H. Hwang and S.-M. Yang, J. Mater. Chem. C, 2013, 1, 426–431 RSC.
- S. A. Maier, Plasmonics: Fundamentals and Applications, 2007 Search PubMed.
- L. Du, D. Tang, G. Yuan, S. Wei and X. Yuan, Appl. Phys. Lett., 2013, 102, 081117 CrossRef.
- T. Y. Jeon, D. J. Kim, S.-G. Park, S.-H. Kim and D.-H. Kim, Nano Convergence, 2016, 3, 1–18 CrossRef.
- T. Y. Jeon, S.-G. Park, D.-H. Kim and S.-H. Kim, Adv. Funct. Mater., 2015, 25, 4681–4688 CrossRef CAS.
- F. S. Ou, M. Hu, I. Naumov, A. Kim, W. Wu, A. M. Bratkovsky, X. Li, R. S. Williams and Z. Li, Nano Lett., 2011, 11, 2538–2542 CrossRef CAS PubMed.
- H. C. Jeon, S. G. Han, S.-G. Park and S.-M. Yang, RSC Adv., 2012, 2, 2334 RSC.
- D. J. Kim, T. Y. Jeon, Y.-K. Baek, S.-G. Park, D.-H. Kim and S.-H. Kim, Chem. Mater., 2016, 28, 1559–1565 CrossRef CAS.
- K. A. Stoerzinger, J. Y. Lin and T. W. Odom, Chem. Sci., 2011, 2, 1435–1439 RSC.
- Y. Sun, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- N. D. Jayram, S. Sonia, S. Poongodi, P. S. Kumar, Y. Masuda, D. Mangalaraj, N. Ponpandian and C. Viswanathan, Appl. Surf. Sci., 2015, 355, 969–977 CrossRef CAS.
- F. Xu, Y. Zhang, Y. Sun, Y. Shi, Z. Wen and Z. Li, J. Phys. Chem. C, 2011, 115, 9977–9983 CAS.
- X. Li, H. K. Lee, I. Y. Phang, C. K. Lee and X. Y. Ling, Anal. Chem., 2014, 86, 10437–10444 CrossRef CAS PubMed.
- H. Häkkinen, Nat. Chem., 2012, 4, 443–455 CrossRef PubMed.
- W. Wang, Y. Yin, Z. Tan and J. Liu, Nanoscale, 2014, 6, 9588 RSC.
- X. Pan, J. Dong, Y. Li, X. Sun, C. Yuan and W. Qian, RSC Adv., 2016, 6, 29586–29591 RSC.
- W. Zhou, A. Hu, S. Bai, Y. Ma and D. Bridges, RSC Adv., 2015, 5, 39103–39109 RSC.
- F. Hidri, N. Sghaier, H. Eloukabi, M. Prat and S. Ben Nasrallah, Phys. Fluids, 2013, 25, 127101 CrossRef.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Nature, 1997, 389, 827–829 CrossRef CAS.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 756–765 CrossRef CAS.
- R. Deegan, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 61, 475–485 CrossRef CAS.
- S.-G. Park, J. H. Moon, S.-K. Lee, J. Shim and S.-M. Yang, Langmuir, 2010, 26, 1468–1472 CrossRef CAS PubMed.
- European Food Safety Authority, EFSA J., 2013, 11, 3231–3238 CrossRef.
- E. Pensa, E. Cortés, G. Corthey, P. Carro, C. Vericat, M. H. Fonticelli, G. Benítez, A. A. Rubert and R. C. Salvarezza, Acc. Chem. Res., 2012, 45, 1183–1192 CrossRef CAS PubMed.
- B. M. Barngrover and C. M. Aikens, J. Phys. Chem. A, 2011, 115, 11818–11823 CrossRef CAS PubMed.
- A. D. McFarland, M. A. Young, J. A. Dieringer and R. P. Van Duyne, J. Phys. Chem. B, 2005, 109, 11279–11285 CrossRef CAS PubMed.
- H. C. Jeon, S.-G. Park, S. Cho and S.-M. Yang, J. Mater. Chem., 2012, 22, 23650 RSC.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546–551 RSC.
- S.-G. Park, S. Y. Lee, S. G. Jang and S.-M. Yang, Langmuir, 2010, 26, 5295–5299 CrossRef CAS PubMed.
- S.-G. Park, J. H. Moon, H. C. Jeon and S.-M. Yang, Soft Matter, 2012, 8, 4567–4570 RSC.
- E. D. B. Santos, E. C. N. L. Lima, C. S. De Oliveira, F. A. Sigoli and I. O. Mazali, Anal. Methods, 2014, 6, 3564–3568 RSC.
- G. N. Xiao and S. Q. Man, Chem. Phys. Lett., 2007, 447, 305–309 CrossRef CAS.
- M. Chen, I. Y. Phang, M. R. Lee, J. K. W. Yang and X. Y. Ling, Langmuir, 2013, 29, 7061–7069 CrossRef CAS PubMed.
- J. H. Kim, T. S. Shim and S. H. Kim, Adv. Mater., 2016, 28, 291–298 CrossRef CAS PubMed.
- H. Hu and R. G. Larson, J. Phys. Chem. B, 2006, 110, 7090–7094 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary videos showing surface wettabilities and supplementary figures showing water contact angle change of alumina surface upon chemisorption of perfluorodecanethiol molecules and enhancement factor calculation. See DOI: 10.1039/c6ra20962a |
| ‡ M. K. Lee and C. W. Mun contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2016 |