Design of a novel wound dressing consisting of alginate hydrogel and simvastatin-incorporated mesoporous hydroxyapatite microspheres for cutaneous wound healing
Weilin Yu†
a,
Ying-Ying Jiang†b,
Tuan-Wei Sunb,
Chao Qib,
Huakun Zhaoa,
Feng Chenb,
Zhongmin Shia,
Ying-Jie Zhu*b,
Daoyun Chen*a and
Yaohua He*a
aDepartment of Orthopedics, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, 600 Yishan Road, Shanghai 200233, China. E-mail: hyhua18930177339@163.com; chendaoyun324309@163.com; Tel: +86-21-24058037
bState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, 1295 Dingxi Road, Shanghai 200050, China. E-mail: y.j.zhu@mail.sic.ac.cn; Tel: +86-21-52412616
First published on 27th October 2016
Abstract
Wound dressings with pro-angiogenic activity are desirable for the rapid healing of full-thickness cutaneous wounds. It is well accepted that simvastatin can stimulate angiogenesis in addition to its lipid-lowering efficacy. However, the construction of a hydrogel-based wound dressing containing simvastatin remains a challenge due to its water-insolubility. In the present study, a novel wound dressing composed of alginate hydrogel (AH) and simvastatin-incorporated mesoporous hydroxyapatite microspheres (S-MHMs) was constructed for the sustained drug release of simvastatin. We first investigated the effect of simvastatin on the angiogenic differentiation of human umbilical vein endothelial cells (HUVECs). The in vitro results revealed that simvastatin significantly promoted the migration and tube formation of HUVECs. Furthermore, the activation of the Akt and Erk signaling pathways was detected in HUVECs upon treatment with simvastatin, and enhanced tube formation was reversed by LY294002 and PD98059, which indicated that both the Akt and Erk signaling pathways were involved in the process of angiogenesis induced by simvastatin. Moreover, simvastatin significantly up-regulated the expression of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF), which play essential roles in angiogenesis. For the in vivo experiments, simvastatin was incorporated into mesoporous hydroxyapatite microspheres (MHMs) synthesized using fructose 1,6-bisphosphate trisodium salt (FBP) as the phosphorous source by the microwave-assisted hydrothermal method. An simvastatin-loaded MHMs (S-MHMs) incorporated alginate hydrogel (S-MHMs/AH) was prepared as a wound dressing to promote the full-thickness cutaneous wound healing. The results demonstrated that S-MHMs/AH significantly enhanced new blood vessel formation and accelerated the reepithelialization of the cutaneous wounds. The present study suggests the potential application of the S-MHMs/AH composite as a novel dressing for wound healing.
1. Introduction
Cutaneous wound healing is a complex programmed sequence of cellular and molecular processes involving inflammation, angiogenesis, migration, proliferation and remodeling.1–4 This dynamic process requires the coordination of epidermal cells, vascular cells, fibroblasts, inflammatory cells and extracellular matrix.5 Many factors, such as ischemia, venous deficiency, infection and diabetes mellitus may adversely affect the healing of cutaneous wounds.6 Disorders in angiogenesis are associated with various types of wound non-healing with the angiogenic impairment of endothelial cells and a reduction of active factors.7,8 Angiogenesis is an essential step during cutaneous wound healing because blood vessels not only deliver oxygen and nutrients to the cells in the wound site but also provide circulating stem cells and active factors critical to wound repair. The reestablishment of the vascular network and perfusion is crucial to accelerate cutaneous wound repair.9Simvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, is widely used in cholesterol-lowering therapy and the prevention of cardiovascular disease.10 In addition to its lipid-lowering properties, it has been suggested that simvastatin could be a potential agent to improve the angiogenic process.11,12 Asai et al. demonstrated that simvastatin could accelerate wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis.13 Activation of the PI3K/Akt pathway may account for the angiogenic effects of simvastatin, including the promotion of new blood vessel formation.14 Moreover, simvastatin has been found to stimulate vascular endothelial growth factor (VEGF) expression through the up-regulation of hypoxia-inducible factor-1α (HIF-1α) and improve wound healing in an experimental model of diabetes.11,15 On account of the angiogenic effect of simvastatin, topical sustainable release of simvastatin may have therapeutic potential in improving the cutaneous wound healing, especially in the case of microvascular dysfunction.
Alginate derived from brown sea algae is an anionic linear polysaccharide composed of 1,4-linked β-D-mannuronate and 1,4-linked α-L-guluronate in varying proportions, which have biocompatibility, non-immunogenicity, biodegradability and antimicrobial activity. Alginate hydrogels are commonly gelated using calcium ions as the cross-linking agent. It has been demonstrated that the alginate hydrogel shows great potential as a wound dressing material due to its high water content, elasticity, permeability and ability to create a moist environment.16–20 Maintenance of a moist or wet wound environment has been shown to promote reepithelialization and reduce scar formation.21 In addition, the hemostatic ability of calcium ions from the calcium alginate hydrogels and the properties of the hydrogel to serve as a matrix for the aggregation of plates and erythrocytes have beneficial effects on wound healing.
In view of the advantages of simvastatin and alginate hydrogel, a simvastatin-incorporated alginate hydrogel is desirable for cutaneous wound healing. However, it is practically impossible to achieve the homogenous distribution of water-insoluble simvastatin in the hydrogel and sustained release of simvastatin into the implantation site. Tangio et al. reported that simvastatin could be water-solubilized in a gelatin hydrogel by grafting gelatin with a L-lactic acid oligomer in the form of polymer micelles.22 Gong et al. reported that the highly hydrophobic curcumin could be solubilized into a thermosensitive hydrogel by being encapsulated in poly(ethyleneglycol)–poly(ε-caprolactone) (PEG–PCL) polymeric micelles with high drug loading and encapsulation efficiency.23 In addition to polymeric micelles such as the L-lactic acid oligomer and PEG–PCL polymer, mesoporous biomaterials are potential drug carriers for the hydrophobic simvastatin because of their mesoporous structure and high specific surface area. In our previous studies, calcium phosphate nanostructured mesoporous microspheres were synthesized successfully using adenosine 5′-triphosphate disodium salt (ATP) or FBP as the phosphorus source through the microwave-assisted hydrothermal method, and these nanostructured microspheres are promising for drug delivery because of their high specific surface area and mesoporous structure.24,25 Benefiting from the merits of high biocompatibility, mesoporous structure and large specific surface area, mesoporous biomaterials present promising application prospects in loading, storage, controlled drug release, and targeted drug delivery.26–29 In addition, porous biopolymer composite hydrogels have advantages such as the enhancement of mechanical strength, higher water uptake and thermal properties.30 It was assumed that mesoporous calcium phosphate microspheres investigated in the present work could not only serve as micelles for homogeneous distribution of water-insoluble simvastatin but also provide high specific surface area and mesoporous structure for high drug loading capacity and sustained drug release.
In the present study, the angiogenic effects of simvastatin on HUVECs were explored by using transwell assay, tube formation assay, qPCR and western blotting. The Akt and Erk signaling pathways were further explored. Mesoporous hydroxyapatite microspheres (MHMs) were synthesized through the microwave-assisted hydrothermal method using fructose 1,6-bisphosphate trisodium salt (FBP) as the phosphorus source. Subsequently, simvastatin-loaded MHMs (S-MHMs) were incorporated into an alginate hydrogel to construct a S-MHMs/AH composite as a novel wound dressing, which was applied in a rodent full-thickness cutaneous defect model. Radiological and histological examinations were conducted to investigate the therapeutic effects of S-MHMs/AH on cutaneous wound healing.
2. Materials and methods
2.1. Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from fresh umbilical cord veins as described previously.31 Cells were cultured at 37 °C in humidified air containing 5% CO2 in complete medium (CM, M199 (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 1% endothelial cell growth supplement/heparin kit (ECGS/H, Promocell) and 1% (v/v) penicillin/streptomycin (Gibco, USA)).2.2. Cell proliferation assay
The effect of simvastatin on the proliferation of HUVECs was assessed using a cell viability assay kit (Cell Counting Kit-8 (CCK-8); Dojindo Molecular Technologies, Inc., Japan). Briefly, cells were seeded in a 96-well plate at an initial density of 3 × 103 cells per well and cultured in CM containing different concentrations of simvastatin (Sigma, USA) for 1, 3 and 5 days. Then, 100 μL of CM containing 10% CCK-8 solution was added to each well at each time point and incubated at 37 °C for 2 h. The absorbance of the samples was measured at 450 nm with a spectrophotometric microplate reader (Bio-Rad 680, USA).2.3. Cell migration and tube formation assays
The effect of simvastatin on the migration of HUVECs was evaluated using a transwell assay. Briefly, 5 × 104 cells were seeded in the upper chamber of a 24-well transwell plate (Corning; pore size = 8 μm). Then, 600 μL of medium containing different concentrations of simvastatin was added to the lower chamber. Eight hours later, the cells from the upper chamber were gently removed with a cotton swab. Subsequently, the cells that migrated to the lower chamber were fixed with 4% paraformaldehyde, stained with 0.5% crystal violet for 15 min and observed with an optical microscope (Leica, Germany). Finally, the crystal violet on the lower surface was solubilized with 500 μL 33% acetic acid solution. The optical density (OD) of the plates was measured at 595 nm in a spectrophotometric microplate reader.For tube formation assay, 100 μL of growth factor-depleted Matrigel (Becton Dickinson, MA) was added to a 48-well plate and allowed to gel for 30 min at 37 °C. Then, the Matrigel was overlaid with a suspension of cells (3 × 104 cells per well) and the cells were treated with different concentrations of simvastatin (0, 0.01, 0.1, 1 or 10 μM). After incubation for 8 h, the tube formation was observed with an optical microscope and the total length of the tube-like structures was outlined and measured using the ImageJ software.
2.4. Western blot assay
For the detection of phosphorylated-Erk (p-Erk) and phosphorylated-Akt (p-Akt), the cells were cultured in the medium containing 1 μM of simvastatin for 0, 5, 15, 30 or 45 min after serum starvation for 24 h. For the detection of HIF-1α and VEGF, the cells were cultured with different concentrations of simvastatin for 48 h. To detect the expression of HIF-1α, CoCl2 (Sigma, USA) was added to the medium with a final concentration of 100 μM. An equal amount of protein from each sample was separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred onto polyvinylidenedifluoride membranes (Millipore, MA, USA). Then, the membranes were incubated with primary antibodies including rabbit anti-human Akt, Erk, p-Erk, p-Akt, HIF-1α and VEGF (Abcam, UK, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000). After incubation with peroxidase-coupled secondary antibodies for 1 h, the membranes were visualized using a chemiluminescence system (Supersignal; Thermo Scientific, Rockford, IL).
1000). After incubation with peroxidase-coupled secondary antibodies for 1 h, the membranes were visualized using a chemiluminescence system (Supersignal; Thermo Scientific, Rockford, IL).
2.5. Erk and Akt inhibitor treatment analysis
The cells were pretreated with a PI3 kinase inhibitor, LY294002 (CST, USA, 50 μM), or the Erk signaling inhibitor PD98059 (CST, USA, 10 μM) for 30 min. Then, the cells were treated with or without simvastatin at the concentration of 1 μM during the tube formation assay. The tube formation activity was estimated in an optical microscope after 8 hours of culture and quantitatively analyzed.2.6. Real-time quantitative reverse transcription PCR (qRT-PCR) analysis
Cells were cultured in the media with different concentrations of simvastatin for 12, 24 and 48 h. Then the cells were processed for RNA isolation, cDNA synthesis, and qRT-PCR. The gene expression level was normalized to that of the housekeeping gene GAPDH and relative gene expression was analyzed using the 2−ΔΔCt method. Each measurement was assessed in triplicate to obtain an average value.2.7. Synthesis and characterization of mesoporous hydroxyapatite microspheres (MHMs)
The mesoporous hydroxyapatite microspheres (MHMs) were synthesized according to a previously reported method.25 In a typical experiment, an aqueous solution (15 mL) containing 0.122 g of fructose 1,6-bisphosphate trisodium salt (FBP, Sangon Biotech, Shanghai) was added dropwise to 30 mL CaCl2 aqueous solution (0.111 g CaCl2) under magnetic stirring. The pH value of the mixed solution was maintained at pH 10 by the slow addition of a 1 M NaOH aqueous solution. The resulting solution was transferred into a 60 mL autoclave, sealed, and heated in a microwave oven (MDS-6, Sineo, China) to 140 °C and maintained at that temperature for 10 min. After cooling to room temperature, the sample was separated by centrifugation, washed twice with deionized water, and dried at 60 °C for 24 h.Scanning electron microscopy (SEM) images and transmission electron microscopy (TEM) images were recorded with a field-emission scanning electron microscope (FE-SEM, FEI Magellan 400, USA) and a transmission electron microscope (TEM, Hitachi H-800, Japan), respectively. The X-ray diffraction (XRD) pattern was recorded using an X-ray diffractometer (Rigaku D/max 2550 V, Cu Kα radiation, λ = 1.54178 Å).
2.8. Preparation of simvastatin-loaded MHMs (S-MHMs) and in vitro simvastatin release assay
The simvastatin loading and release experiment was performed as follows. The MHMs (100 mg) were dispersed into an ethanol solution of simvastatin (20 mL, 5.0 mg mL−1). The suspension was shaken in a sealed vessel at a constant rate (120 rpm) at 37 °C for 24 h, followed by centrifugation and freeze-drying at −20 °C for 24 h to obtain the S-MHMs. For the simvastatin release assay, the S-MHMs (10 mg) was immersed into phosphate buffered saline (PBS, 20 mL) at 37 °C with constant shaking (120 rpm). The drug release medium (0.85 mL) was extracted and measured by UV-Vis absorption spectroscopy at a wavelength of 238 nm at given time intervals and replaced with the same volume of PBS.2.9. Preparation of S-MHMs incorporated alginate hydrogel (S-MHMs/AH) and in vitro release assay
For the preparation of S-MHMs/AH, 0.600 g of sodium alginate was dissolved into 30 mL deionized water, and then 0.360 g of S-MHMs was dispersed in sodium alginate solution ultrasonically and mixed together under magnetical stirring for 20 min. Then, 0.85 mL S-MHMs/sodium alginate hybrid solution was transferred into the 24-well plate. After that, CaCl2 aqueous solution (20 mg mL−1) was sprayed over the surface of sodium alginate solution and S-MHMs/sodium alginate hybrid solution to ensure complete gelatinization. The simvastatin release assay of the S-MHMs/AH was performed as described above.2.10. Evaluation of healing of full-thickness skin defects in rodents
For the evaluation of neovascularization, the immunohistochemical (IHC) staining for CD31 was first performed. The immunofluorescence (IF) staining of paraffin sections with CD31 and α-SMA antibodies was performed to evaluate the formation of the mature vessels. The mature vessels were indicated as CD31 and α-SMA double-positive vascular structures. Quantification of newly formed vessels and mature vessels were conducted by counting in three random fields per section between wound edges using Image-Pro plus 6.
2.11. Statistical analysis
All data were expressed as the mean ± standard deviation (SD) and were analyzed with SPSS software. One-way analysis of variance and Student–Newman–Keuls post hoc tests were used to determine the level of significance, with p values less than 0.05 being considered statistically significant.3. Results
3.1. Effect of simvastatin on cell proliferation, migration and tube formation
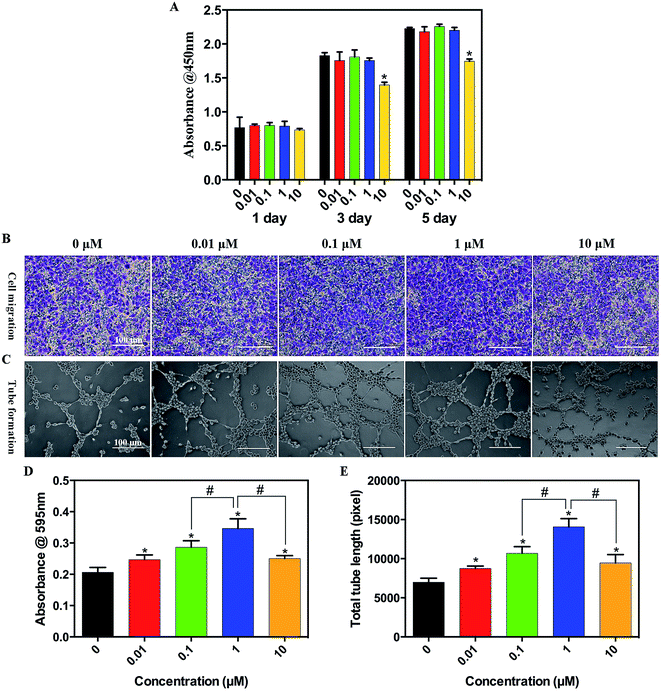
The effect of simvastatin on the proliferation of HUVECs is shown in Fig. 1A. The CCK-8 assay showed that simvastatin less than or equal to 1 μM did not affect cell proliferation compared with the control group, whereas cell proliferation was significantly inhibited when the concentration of simvastatin reached 10 μM.A transwell assay was used to evaluate the migration of HUVECs (Fig. 1B and D). The migration capacities of cells cultured in the medium supplemented with simvastatin were significantly increased compared with the control group, particularly at the concentration of 1 μM. As shown in Fig. 1C and E, after incubation on Matrigel for 8 h, the tube formation capacity of HUVECs was significantly enhanced by simvastatin, especially in the 1 μM group.
3.2. The activation of Akt and Erk signaling pathways
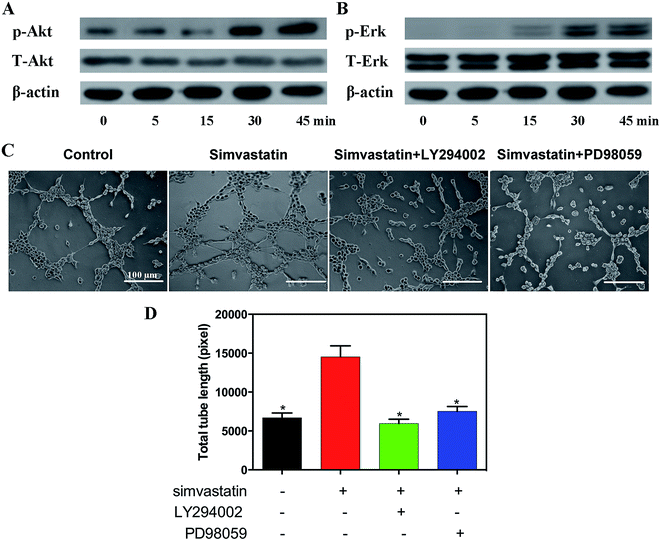
To explore the effect of simvastatin on the activation of Akt and Erk pathways involved in the angiogenesis process, the related protein was analyzed by western blotting after 0, 5, 15, 30 and 45 min of culture. As shown in Fig. 2A and B, phosphorylation of Akt was detected 30 min after exposure to simvastatin and increased to 45 min, and phosphorylation of Erk was observed after 15 min of exposure and increased to 45 min.To further confirm the roles of the Akt and Erk signaling pathways in simvastatin-induced angiogenesis, we used the PI3K inhibitor LY294002 and the Erk inhibitor PD98059. Simvastatin-induced tube formation was significantly reversed by LY294002 and PD98059 (Fig. 2C and D).
3.3. VEGF and HIF-1α expression
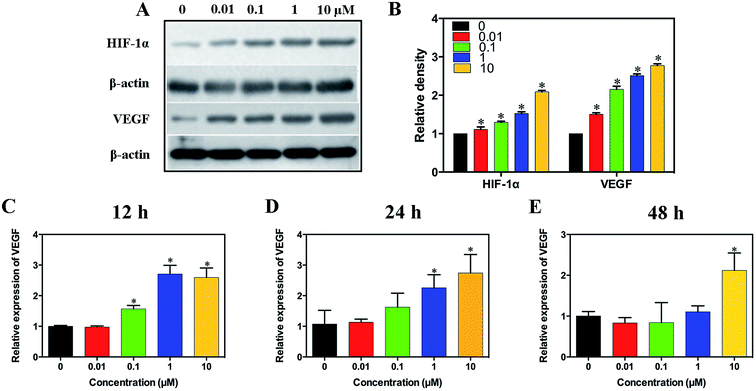
The effect of simvastatin on HIF-1α and VEGF expression was first determined by the western blot assay. As shown in Fig. 3A and B, simvastatin increased the expression of HIF-1α and VEGF in a concentration dependent manner. As shown in Fig. 3C and D, the gene expression of VEGF was significantly improved by simvastatin at the concentrations of 0.1, 1 and 10 μM after 12 hours of culture, and was significantly improved by simvastatin at the concentrations of 1 and 10 μM after 24 hours of culture. However, the gene expression of VEGF was significantly increased only at the concentration of 10 μM after 48 hours of culture (Fig. 3E).3.4. Characterization and in vitro drug release
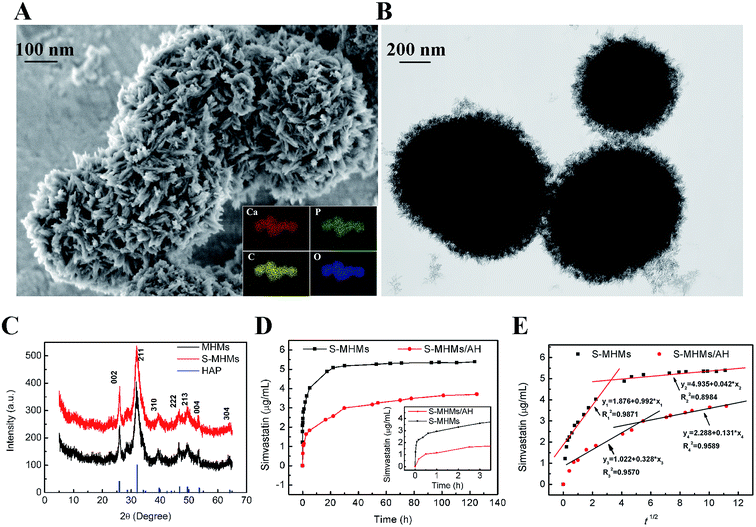
As shown in Fig. 4A and B, the MHMs consisted of hydroxyapatite nanosheets/nanorods that were hierarchically assembled into nanostructured mesoporous microspheres, and the agglomeration of microspheres was observed. As shown in Fig. 4C, the XRD pattern indicated that the diffraction peaks of MHMs and S-MHMs could be indexed to a single phase of hydroxyapatite with a hexagonal structure (Ca5(PO4)3(OH), JCPDS no. 09-0432).The simvastatin release from the S-MHMs and S-MHMs/AH drug delivery system is shown in Fig. 4D. For the S-MHMs/AH drug release system, the drug release process mainly consisted of two release stages. The first stage was the rapid drug release in the very first several hours. Then, the S-MHMs/AH maintained a sustained drug release in the second stage, and the drug release rate of the S-MHMs/AH gradually decreased and achieved a release balance of simvastatin. In contrast, the drug release rate of the S-MHMs drug delivery system was much faster. The drug release of the S-MHMs/AH lasted much longer than the S-MHMs, exhibiting a superior drug release performance. Fig. 4E shows the relationship between the cumulative amount of the released drug and the square root of release time. From Fig. 4E, one can see that there was a linear relationship between the cumulative amount of released drug and the square root of release time.
3.5. Wound healing effect of S-MHMs/AH in the full-thickness cutaneous defect model
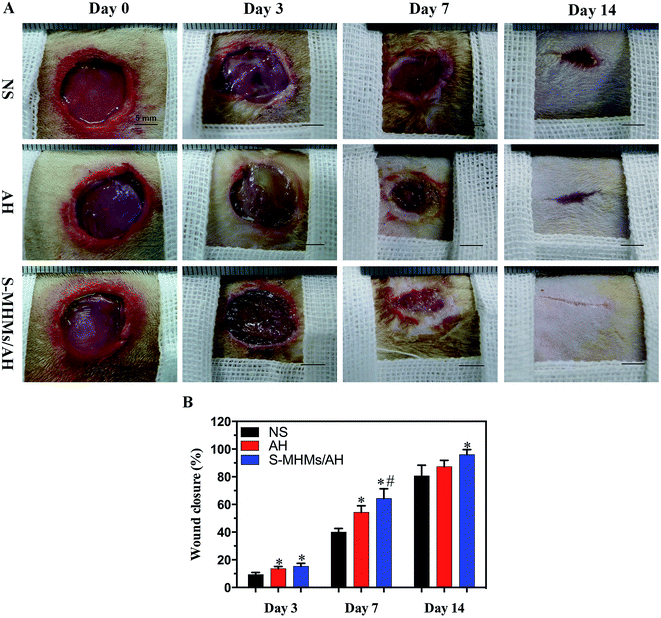
Fig. 5A shows the gross appearance of cutaneous defects after treatment with NS, AH and S-MHMs/AH. No adverse effects such as inflammation or infection were observed in the wounds throughout the post-operation period. The wound closure rate at days 3, 7 and 14 in the NS, AH and S-MHMs/AH groups was analyzed as a percentage of the reduction in wound area (Fig. 5B). The wound size for each group became smaller with time. In the S-MHMs/AH group, the wound areas were significantly smaller than the NS group at days 3, 7 and 14. At days 3 and 7, the wound areas in the AH group were significantly smaller than those in the NS group. Moreover, the areas of defects treated with S-MHMs/AH were significantly smaller than those treated with AH at day 7.3.6. Micro-CT evaluation of neovascularization
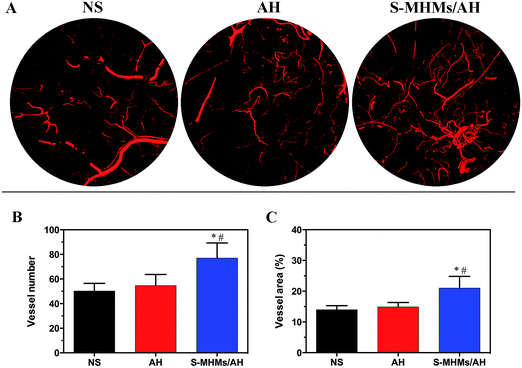
Three-dimensional micro-CT reconstructions revealed new vessel formation in the cutaneous defects at day 14. As shown in Fig. 6A, the S-MHMs/AH group displayed a better neovascularization than the AH and NS groups. According to the quantification of the vascular network, there was a significantly higher blood vessel number and larger blood vessel area in the cutaneous defects treated with S-MHMs/AH than in the defects treated with AH or NS (Fig. 6B and C).3.7. Histological, IHC and IF evaluation
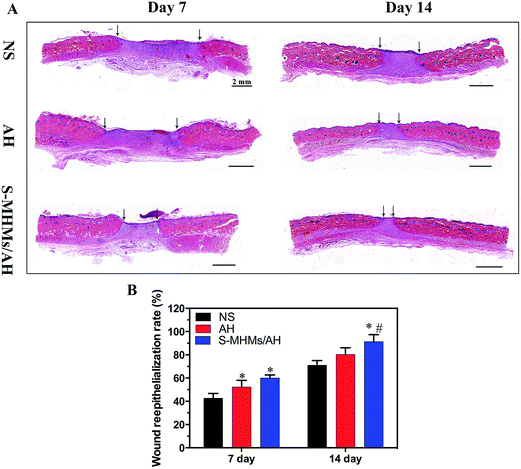
The degree of cutaneous wound healing was further determined by the cutaneous wound reepithelialization rate and collagen maturity. As shown in Fig. 7A and B, the reepithelialization degree in the defects treated with S-MHMs/AH was significantly enhanced compared with the defects treated with NS at day 7 post-operation, whereas the difference between AH and S-MHMs/AH was not significant. At day 14, the reepithelialization rate of the S-MHMs/AH group was faster than in the NS and AH groups. The histological analytic results of collagen deposition and maturity were determined by the Masson's trichrome staining (Fig. 8). More collagen deposition was observed in the defects treated with S-MHMs/AH than those treated with AH or NS at days 7 and 14 post-operation. Notably, there were extensive waxy collagen fibers with an ordered arrangement in the S-MHMs/AH group.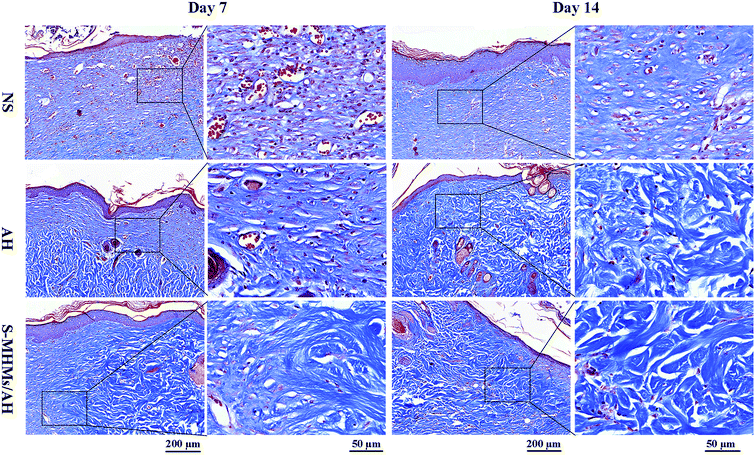 | ||
| Fig. 8 Masson's trichrome stained sections of the defects treated with NS, AH and S-MHMs/AH at 7 and 14 days post-operation, showing collagen deposition and maturity. | ||
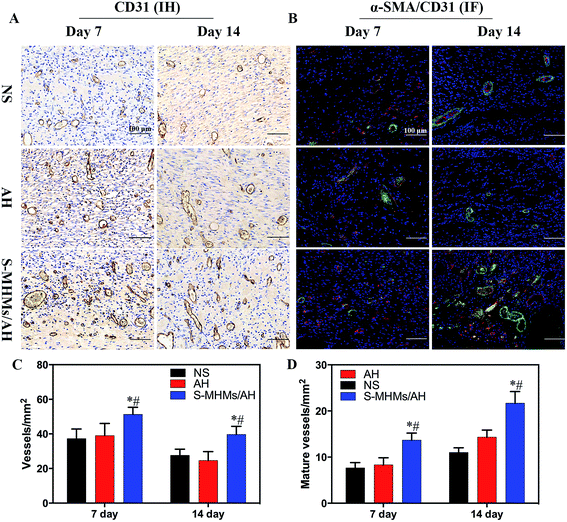
The neovascularization in the defects treated with NS, AH and S-MHMs/AH was further determined by IHC staining for the endothelial marker CD31 (Fig. 9A and C) and IF co-staining for CD31 and α-SMA (Fig. 9B and D). The IHC results revealed that the number of newly formed vessels in the cutaneous defects decreased from day 7 to day 14 post-operation, and the vessel density in S-MHMs/AH-treated defects was significantly higher than the NS- and AH-treated defects at days 7 and 14. As shown in Fig. 9B, the mature vessels were characterized by co-staining against CD31 (red) and α-SMA (green). Quantitative analysis revealed that the number of mature vessels in the cutaneous defects increased from day 7 to day 14, and the density of mature blood vessel of the S-MHMs/AH group was significantly higher than the NS and AH groups at days 7 and 14 (Fig. 9D).
4. Discussion
In the present study, the angiogenic activity of simvastatin and the underlying mechanism was investigated firstly. To investigate the potential of simvastatin in improving cutaneous wound healing, a sustained simvastatin drug release system composed of simvastatin-loaded mesoporous hydroxyapatite microspheres and alginate hydrogel (S-MHMs/AH) was constructed. The S-MHMs/AH showed a sustained release of simvastatin. Moreover, the in vivo results demonstrated significantly enhanced neovascularization and accelerated cutaneous wound healing for the skin defects treated with S-MHMs/AH compared with the defects treated with NS or AH.4.1. Angiogenic effect of simvastatin
Simvastatin is well known for its lipid-lowering properties and beneficial effects in cardiovascular diseases. In addition to its lipid-lowering properties, simvastatin exerts its effects on angiogenesis and protects against ischemic injury.11,12 It has been demonstrated that the angiogenic effects of simvastatin are mainly mediated through the PI3-kinase/Akt pathway.13,14 Consistent with the above results, capillary morphogenesis and migration of HUVECs were significantly enhanced by simvastatin, especially at the concentration of 1 μM. The cytotoxicity caused by simvastatin at 10 μM might explain the impaired tube formation and migration capacities of HUVECs. Moreover, an up-regulation of the phosphorylation of Akt protein was also observed in this study. The PI3-kinase/Akt pathway is involved in multiple cellular processes, including cell proliferation, migration, adhesion and survival. The activation of the PI3K/Akt/mTOR/p70 S6 kinase pathway mediates VEGF-induced endothelial cell survival, NO production and migration.14 Furthermore, we examined the role of the Erk pathway in the angiogenesis process of HUVECs induced by simvastatin. The results revealed that the Erk signaling pathway was phosphorylated in HUVECs treated with simvastatin. More importantly, the capillary morphogenesis of HUVECs was reversed when the cells were treated with LY294002 and PD98059, indicating that both the Akt and Erk signaling pathways are involved in the angiogenesis process induced by simvastatin. To the best of our knowledge, the role of Erk signaling pathway in simvastatin-induced angiogenesis have not been reported previously. It is well known that the Raf/Mek/Erk signaling cascade is involved in the angiogenesis process.32 It has been demonstrated that the Erk signaling pathway plays an important role in therapeutic drugs promoting angiogenesis during wound repair.33–35 Taken together, the coactivation of Akt and Erk signaling by simvastatin contributed to the angiogenic differentiation of HUVECs. Whether other signaling pathways are involved in the angiogenesis process induced by simvastatin needs further investigation.We also investigated the effect of simvastatin on the expression of HIF-1α and VEGF. As was expected, simvastatin up-regulated the protein expression of HIF-1α and VEGF in a concentration-dependent manner. On one hand, the activation of the PI3k/Akt/mTOR/p70 S6 kinase pathway can promote angiogenesis by up-regulating the translation of HIF-1α and the subsequent HIF-1α dependent expression and secretion of VEGF.36,37 On the other hand, the Erk signaling pathway is not only involved in regulation of HIF-1α translation but also its transcriptional activity. Erk phosphorylated the co-activator CBP/p300 and increased the formation of the HIF-1α/p300 complex, and thus increased its transcriptional activity.38 The angiogenic activity of simvastatin suggests its potential application as a therapeutic drug for the acceleration of cutaneous wound healing.
4.2. Simvastatin drug delivery and release
The present study demonstrated that the S-MHMs/AH could continuously release water-insoluble simvastatin with biological activity. The MHMs showed a high drug-loading capacity due to the mesoporous structure and high specific surface area, which make it an excellent nanocarrier for the delivery of simvastatin. However, the therapeutic efficacy of simvastatin is limited due to its poor solubility in aqueous media.22 With the aid of MHMs, simvastatin-loaded MHMs acted as micelles that could be homogenously dispersed and immobilized in the matrix of alginate hydrogel. To the best of our knowledge, this is the first report using hydroxyapatite nanostructured mesoporous microspheres as the nanocarrier for the water-insoluble drug in a hydrogel system. Zeng et al. demonstrated that the bioglass powder with an average particle size of 20 μm was successfully incorporated into an agarose–alginate composite hydrogel homogenously, and the calcium ions released from bioglass powder further cross-linked the alginate polymer chains.39 Similarly, the calcium ions released from MHMs contribute to the crosslinking of the alginate hydrogel, which may help the hydrogel to maintain its shape and prevent alginate from leaking out. Nanostructured materials of hydroxyapatite have been used as drug carriers for decades40,41 and the drug release kinetics are dependent on the specific properties of the drug and the morphology of hydroxyapatite nanostructures.41 The as-prepared S-MHMs/AH drug delivery system exhibited a relatively rapid drug release profile in the first several hours, which may be attributed to the release of weakly adsorbed simvastatin molecules on the surface of MHMs.25 This rapid drug release at the early stage is usually desirable because the released drug can reach the effective concentration required for the treatment in a short period of time. Then, the S-MHMs/AH drug delivery system maintained a sustained drug release in the second release stage, and the drug release rate of the S-MHMs/AH drug delivery system gradually decreased and achieved a release balance of simvastatin. The drug release of the S-MHMs/AH lasted much longer than the S-MHMs, exhibiting a superior drug release performance. In addition, there was a linear relationship between the cumulative amount of released drug and the square root of release time, indicating that the drug release from the S-MHMs/AH drug delivery system is governed by a diffusion process, which is consistent with the Higuchi model.42 The combination of alginate hydrogel greatly slowed down the drug release rate of the S-MHMs drug delivery system and prolonged the total drug release time. It is assumed that the alginate hydrogel acts as a buffering system to curb the rapid release of simvastatin. The high porosity and water content of the hydrogels allow the entrapment and immobilization of therapeutic agents such as drugs and growth factors, which are then gradually released to the implant site.43 The combination of S-MHMs and alginate hydrogel forms a sustained drug release complex, in which the S-MHMs act as a drug-reservoir and the alginate hydrogel works as the buffering zone and drug diffusion network.4.3. Effect of S-MHMs/AH on wound healing
The S-MHMs/AH composite significantly accelerated the healing of cutaneous defects, accompanied with enhanced neovascularization. A moist local environment provided by alginate dressings is favorable for wound healing.44 It was demonstrated that the maintenance of a physiological moist environment would promote granulation tissue formation, fast reepithelialization and final wound healing.43 In addition, Wang et al. demonstrated that calcium alginate improved the synthesis of type I collagen and increased the ratio of collagen I/III during wound healing.45 To date, various alginate-based wound dressings have been developed to promote wound healing and are commercially available.Neovascularization, the formation of new capillaries from the pre-existing vascular network, is an essential step of cutaneous wound healing, especially for ischemic chronic wounds. The formation of the vascular network facilitates the transportation of oxygen and nutrients, removal of cellular metabolic by-products and trafficking of stem cells, which are critical for wound repair.46 The results of micro-CT and histological examinations showed that the skin defects treated with S-MHMs/AH significantly enhanced the formation of new blood vessels compared with the defects treated with AH or NS. We speculated that the sustained release of simvastatin from S-MHMs/AH significantly enhanced the formation of new blood vessels, which resulted in a sufficient supply of nutrients, oxygen and growth factors as well as the migration of stem or progenitor cells. The above processes further improved wound healing with accelerated reepithelialization and collagen deposition. Zhao et al. reported that wound dressings composed of Cu-doped borate bioactive glass microfibers promoted the skin defect repair by stimulating angiogenesis.47 During the wound healing, the up-regulation of VEGF plays a crucial role in the angiogenesis process. It has been demonstrated that VEGF has pleiotropic effects on tissue repair via neovascularization, reepithelialization and regulation of extracellular matrix.48 Bitto et al. demonstrated that simvastatin enhances the production of VEGF and ameliorates impaired wound healing.11 Likewise, the S-MHMs/AH might enhance the expression of VEGF and VEGF-mediated angiogenesis during wound healing.
Taken together, the moist environment provided by the alginate hydrogel and the angiogenic effect from the sustained release of simvastatin contributed to the accelerated healing of cutaneous defects. However, one limitation of the present study is the use of the normal rodent full-thickness cutaneous defect model. In further studies, we plan to use animal models with impaired angiogenesis and refractory wound lesions such as diabetic mice, which may be more persuasive to manifest the superiority of the S-MHMs/AH as a wound dressing.
5. Conclusions
Our results demonstrated that simvastatin could stimulate angiogenesis and both the Akt and Erk signaling pathways were involved in the angiogenesis process induced by simvastatin, followed by the up-regulation of HIF-1α and HIF-1α-mediated VEGF expression. Furthermore, S-MHMs/AH as a novel wound dressing was constructed, in which water-insoluble simvastatin was successfully incorporated into the alginate hydrogel with the aid of MHMs as the drug nanocarrier and showed a sustained drug release. Finally, we demonstrated that S-MHMs/AH accelerated cutaneous defect healing characterized with enhanced angiogenesis, more collagen deposition and faster reepithelialization, which bodes well for the potential application of S-MHMs/AH as a novel wound dressing.Acknowledgements
Financial support from the National High-Tech Research and Development Program of China (863-Project, No. 2015AA020316), National Natural Science Foundation of China (81271961, 81572106, 81271998, 51472259) and the Science and Technology Commission of Shanghai, China (15ZR1431900, 15JC1491001) is gratefully acknowledged.References
- M. B. Dreifke, A. A. Jayasuriya and A. C. Jayasuriya, Mater. Sci. Eng., C, 2015, 48, 651–662 CrossRef CAS PubMed.
- L. Braiman-Wiksman, I. Solomonik, R. Spira and T. Tennenbaum, Toxicol. Pathol., 2007, 35, 767–779 CrossRef PubMed.
- G. Broughton II, J. E. Janis and C. E. Attinger, Plast. Reconstr. Surg., 2006, 117, 12S–34S CrossRef PubMed.
- A. J. Singer and R. A. Clark, N. Engl. J. Med., 1999, 341, 738–746 CrossRef CAS PubMed.
- C. L. Liu, J. C. Tam, A. J. Sanders, C. H. Ko, K. P. Fung, P. C. Leung, K. G. Harding, W. G. Jiang and C. B. Lau, Wound Repair Regen., 2013, 21, 579–587 CrossRef PubMed.
- A. Medina, P. G. Scott, A. Ghahary and E. E. Tredget, J. Burn Care Rehabil., 2005, 26, 306–319 CrossRef PubMed.
- M. G. Tonnesen, X. Feng and R. A. Clark, J. Invest. Dermatol. Symp. Proc., 2000, 5, 40–46 CrossRef CAS PubMed.
- F. Liu, D. D. Chen, X. Sun, H. H. Xie, H. Yuan, W. Jia and A. F. Chen, Diabetes, 2014, 63, 1763–1778 CrossRef CAS PubMed.
- G. K. Kolluru, S. C. Bir and C. G. Kevil, Int. J. Vasc. Med., 2012, 2012, 918267 Search PubMed.
- B. W. Karlson, O. Wiklund, M. K. Palmer, S. J. Nicholls, P. Lundman and P. J. Barter, Eur. Heart J.: Cardiovasc. Pharmacother., 2016, 2, 212–217 CrossRef PubMed.
- A. Bitto, L. Minutoli, D. Altavilla, F. Polito, T. Fiumara, H. Marini, M. Galeano, M. Calo, P. Lo Cascio, M. Bonaiuto, A. Migliorato, A. P. Caputi and F. Squadrito, Pharmacol. Res., 2008, 57, 159–169 CrossRef CAS PubMed.
- M. F. El-Azab, R. M. Hazem and Y. M. Moustafa, Eur. J. Pharmacol., 2012, 690, 31–41 CrossRef CAS PubMed.
- J. Asai, H. Takenaka, S. Hirakawa, J. Sakabe, A. Hagura, S. Kishimoto, K. Maruyama, K. Kajiya, S. Kinoshita, Y. Tokura and N. Katoh, Am. J. Pathol., 2012, 181, 2217–2224 CrossRef CAS PubMed.
- Y. Kureishi, Z. Luo, I. Shiojima, A. Bialik, D. Fulton, D. J. Lefer, W. C. Sessa and K. Walsh, Nat. Med., 2000, 6, 1004–1010 CrossRef CAS PubMed.
- A. Nishimoto-Hazuku, T. Hirase, N. Ide, Y. Ikeda and K. Node, J. Cardiovasc. Pharmacol., 2008, 51, 267–273 CrossRef CAS PubMed.
- B. Sarker, R. Singh, R. Silva, J. A. Roether, J. Kaschta, R. Detsch, D. W. Schubert, I. Cicha and A. R. Boccaccini, PLoS One, 2014, 9, e107952 Search PubMed.
- R. Pereira, A. Carvalho, D. C. Vaz, M. H. Gil, A. Mendes and P. Bartolo, Int. J. Biol. Macromol., 2013, 52, 221–230 CrossRef CAS PubMed.
- Z. Gu, H. Xie, C. Huang, L. Li and X. Yu, Int. J. Biol. Macromol., 2013, 58, 121–126 CrossRef CAS PubMed.
- H. E. Thu, M. H. Zulfakar and S. F. Ng, Int. J. Pharm., 2012, 434, 375–383 CrossRef CAS PubMed.
- C. H. Goh, P. W. Heng, E. P. Huang, B. K. Li and L. W. Chan, J. Antimicrob. Chemother., 2008, 62, 105–108 CrossRef CAS PubMed.
- J. P. Junker, R. A. Kamel, E. J. Caterson and E. Eriksson, Adv. Wound Care, 2013, 2, 348–356 CrossRef PubMed.
- T. Tanigo, R. Takaoka and Y. Tabata, J. Controlled Release, 2010, 143, 201–206 CrossRef CAS PubMed.
- C. Gong, Q. Wu, Y. Wang, D. Zhang, F. Luo, X. Zhao, Y. Wei and Z. Qian, Biomaterials, 2013, 34, 6377–6387 CrossRef CAS PubMed.
- C. Qi, Y. J. Zhu, X. Y. Zhao, B. Q. Lu, Q. L. Tang, J. Zhao and F. Chen, Chem.–Eur. J., 2013, 19, 981–987 CrossRef CAS PubMed.
- C. Qi, Y. J. Zhu and F. Chen, Chem.–Asian J., 2013, 8, 88–94 CrossRef CAS PubMed.
- L. Chen, X. She, T. Wang, L. He, S. Shigdar, W. Duan and L. Kong, Nanoscale, 2015, 7, 14080–14092 RSC.
- Y. Li and J. Shi, Adv. Mater., 2014, 26, 3176–3205 CrossRef CAS PubMed.
- W. R. Erwin, H. F. Zarick, E. M. Talbert and R. Bardhan, Energy Environ. Sci., 2016, 9, 1577–1601 CAS.
- V. Malgras, Q. Ji, Y. Kamachi, T. Mori, F.-K. Shieh, K. C. W. Wu, K. Ariga and Y. Yamauchi, Bull. Chem. Soc. Jpn., 2015, 88, 1171–1200 CrossRef CAS.
- E. A. Naumenko, I. D. Guryanov, R. Yendluri, Y. M. Lvova and R. F. Fakhrullin, Nanoscale, 2016, 8, 7257–7271 RSC.
- V. Marin, G. Kaplanski, S. Gres, C. Farnarier and P. Bongrand, J. Immunol. Methods, 2001, 254, 183–190 CrossRef CAS PubMed.
- J. D. Hood, R. Frausto, W. B. Kiosses, M. A. Schwartz and D. A. Cheresh, J. Cell Biol., 2003, 162, 933–943 CrossRef CAS PubMed.
- G. Han, F. Li, P. Ten Dijke and X. J. Wang, Am. J. Pathol., 2011, 179, 1768–1779 CrossRef CAS PubMed.
- S. M. Chim, A. Qin, J. Tickner, N. Pavlos, T. Davey, H. Wang, Y. Guo, M. H. Zheng and J. Xu, J. Biol. Chem., 2011, 286, 22035–22046 CrossRef CAS PubMed.
- W. S. Kim, Y. J. Yang, H. G. Min, M. G. Song, J. S. Lee, K. Y. Park, J. J. Kim, J. H. Sung, J. S. Choi and H. J. Cha, Pharmacology, 2010, 85, 68–76 CrossRef CAS PubMed.
- J. Karar and A. Maity, Front. Mol. Neurosci., 2011, 4, 51 CAS.
- K. Shen, L. Ji, C. Gong, Y. Ma, L. Yang, Y. Fan, M. Hou and Z. Wang, Biochem. Pharmacol., 2012, 84, 784–792 CrossRef CAS PubMed.
- N. Sang, D. P. Stiehl, J. Bohensky, I. Leshchinsky, V. Srinivas and J. Caro, J. Biol. Chem., 2003, 278, 14013–14019 CrossRef CAS PubMed.
- Q. Zeng, Y. Han, H. Li and J. Chang, J. Mater. Chem. B, 2015, 3, 8856–8864 RSC.
- D. Li, J. He, X. Huang, J. Li, H. Tian, X. Chen and Y. Huang, RSC Adv., 2015, 5, 30920–30928 RSC.
- B. Palazzo, M. Iafisco, M. Laforgia, N. Margiotta, G. Natile, C. L. Bianchi, D. Walsh, S. Mann and N. Roveri, Adv. Funct. Mater., 2007, 17, 2180–2188 CrossRef CAS.
- J. Andersson, J. Rosenholm, A. S. Areva and M. Lindén, Chem. Mater., 2004, 16, 4160–4167 CrossRef CAS.
- K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- D. Queen, H. Orsted, H. Sanada and G. Sussman, Int. Wound J., 2004, 1, 59–77 CrossRef PubMed.
- T. Wang, Q. S. Gu, J. Zhao, J. C. Mei, M. Z. Shao, Y. Pan, J. Zhang, H. S. Wu, Z. Zhang and F. Liu, Int. J. Clin. Exp. Pathol., 2015, 8, 6636–6645 Search PubMed.
- P. Carmeliet, Nature, 2005, 438, 932–936 CrossRef CAS PubMed.
- S. Zhao, L. Li, H. Wang, Y. Zhang, X. Cheng, N. Zhou, M. N. Rahaman, Z. Liu, W. Huang and C. Zhang, Biomaterials, 2015, 53, 379–391 CrossRef CAS PubMed.
- S. G. Keswani, S. Balaji, L. D. Le, A. Leung, J. K. Parvadia, J. Frischer, S. Yamano, N. Taichman and T. M. Crombleholme, Wound Repair Regen., 2013, 21, 554–562 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |