Polyhedron Cu2O@Ag composite microstructures: synthesis, mechanism analysis and structure-dependent SERS properties
Abstract
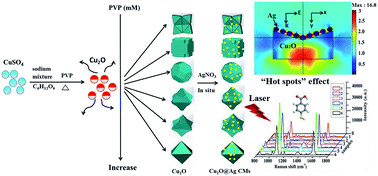
Composite microstructures consisting of Cu2O polyhedrons and Ag nanoparticles (Cu2O@Ag CMs) were successfully prepared through a modified reduction method. In the synthesis process of the Cu2O crystals, by modulating the concentrations of surfactant poly(vinyl pyrrolidone), the as-prepared Cu2O polyhedrons with diverse morphologies displayed a unique shape evolution which clearly revealed their growth mechanism. By in situ adding AgNO3 into the Cu2O solution, Cu2O@Ag CMs were synthesized and their surface-enhanced Raman scattering (SERS) performances were evaluated with the help of labeling with 4-mercaptobenzoic acid molecules. The experimental and simulated results shown that the Cu2O@Ag CMs exhibit structure-dependent SERS characteristics, i.e., their SERS enhancements closely relied on the morphologies of the Cu2O polyhedrons, and the sizes and distributions of the Ag nanoparticles. Especially, under the optimized synthesis conditions, the as-prepared concave trisoctahedron Cu2O@Ag CMs have a superior SERS activity with an enhancement factor of 3.21 × 106. This shows that the synthesized Cu2O@Ag CMs could have potential applications in chemical and biological fields.

 Please wait while we load your content...
Please wait while we load your content...