Nickel and cobalt nanoparticles modified hollow mesoporous carbon microsphere catalysts for efficient catalytic reduction of widely used dyes†
Abstract
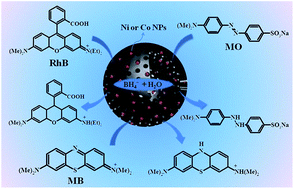
Recently, with increasing consciousness of environmental protection, Chemists are focusing on green chemistry, which allows the transformation of highly toxic organic wastes into reusable or low-toxicity compounds under mild reaction conditions. In this work, hollow mesoporous carbon microspheres (h-MCM) with an ultrahigh surface area were synthesized by a co-sol–emulsion–gel method and were used as supports for Ni and Co nanoparticles (NPs). The prepared nanocatalysts (Ni/h-MCM and Co/h-MCM) have high Ni and Co NPs dispersion on the surface of the h-MCM as well as in its hollow core. The prepared catalysts exhibit a high surface area, mesoporous shell and an accessible interior space. These properties are beneficial for enhancing the catalytic activity of the prepared catalysts. In the catalytic reduction of methylene blue, methyl orange, and rhodamine B, the obtained Ni/h-MCM and Co/h-MCM exhibited excellent catalytic activity as compared with other reported catalysts. This work may promote the design of non-precious metal based nanocatalysts for environmental catalysis.


 Please wait while we load your content...
Please wait while we load your content...